Acidic pH is essential for maintaining mast cell secretory granule homeostasis
- PMID: 28492555
- PMCID: PMC5584528
- DOI: 10.1038/cddis.2017.206
Acidic pH is essential for maintaining mast cell secretory granule homeostasis
Abstract
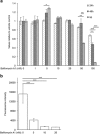
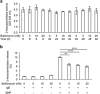
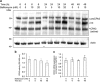
It has been recognized for a long time that the secretory granules of mast cells are acidic, but the functional importance of maintaining an acidic pH in the mast cell granules is not fully understood. Here we addressed this issue by examining the effects of raising the pH of the mast cell secretory granules. Mast cells were incubated with bafilomycin A1, an inhibitor of the vacuolar-type ATPase proton pump. Supporting a role of vacuolar-type ATPase in mast cell granule acidification, bafilomycin A1 treatment caused a robust increase in granule pH. This was accompanied by marked effects on mast cell granules, including swelling and acquisition of vacuole-like morphology. Moreover, bafilomycin A1 caused extensive, yet selective effects on the granule content. These included aberrant processing of pro-carboxypeptidase A3 and a reduction in the level of intracellular histamine, the latter being accompanied by an increase in extracellular histamine. In contrast, the storage of β-hexosaminidase, a prototype lysosomal hydrolase known to be stored in mast cell granules, was not affected by abrogation of granule acidification. Moreover, bafilomycin A1 caused a reduction of tryptase enzymatic activity and appearance of tryptase degradation products. Tryptase inhibition prevented the formation of such degradation products, suggesting that the pH elevation causes tryptase to undergo autoproteolysis. Taken together, our findings reveal that mast cell secretory granule homeostasis is critically dependent on an acidic milieu.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Distorted secretory granule composition in mast cells with multiple protease deficiency.J Immunol. 2013 Oct 1;191(7):3931-8. doi: 10.4049/jimmunol.1301441. Epub 2013 Aug 23. J Immunol. 2013. PMID: 23975861
-
A role for serglycin proteoglycan in granular retention and processing of mast cell secretory granule components.FEBS J. 2006 Nov;273(21):4901-12. doi: 10.1111/j.1742-4658.2006.05489.x. Epub 2006 Sep 28. FEBS J. 2006. PMID: 17010166
-
Polyamines are present in mast cell secretory granules and are important for granule homeostasis.PLoS One. 2010 Nov 30;5(11):e15071. doi: 10.1371/journal.pone.0015071. PLoS One. 2010. PMID: 21151498 Free PMC article.
-
Vesicular Ca(2+) mediates granule motion and exocytosis.Cell Calcium. 2012 Mar-Apr;51(3-4):338-41. doi: 10.1016/j.ceca.2011.12.009. Epub 2012 Jan 4. Cell Calcium. 2012. PMID: 22222091 Review.
-
Mast cell proteoglycans.J Histochem Cytochem. 2012 Dec;60(12):950-62. doi: 10.1369/0022155412458927. Epub 2012 Aug 16. J Histochem Cytochem. 2012. PMID: 22899859 Free PMC article. Review.
Cited by
-
The regulatory role and mechanism of mast cells in tumor microenvironment.Am J Cancer Res. 2024 Jan 15;14(1):1-15. doi: 10.62347/EZST5505. eCollection 2024. Am J Cancer Res. 2024. PMID: 38323271 Free PMC article. Review.
-
Omeprazole inhibits IgE-mediated mast cell activation and allergic inflammation induced by ingested allergen in mice.J Allergy Clin Immunol. 2020 Oct;146(4):884-893.e5. doi: 10.1016/j.jaci.2020.02.032. Epub 2020 Mar 16. J Allergy Clin Immunol. 2020. PMID: 32194041 Free PMC article.
-
Biogenesis and homeostasis of mast cell lysosome related secretory granules.Front Cell Dev Biol. 2025 May 23;13:1603999. doi: 10.3389/fcell.2025.1603999. eCollection 2025. Front Cell Dev Biol. 2025. PMID: 40486912 Free PMC article. Review.
-
Mefloquine causes selective mast cell apoptosis in cutaneous mastocytosis lesions by a secretory granule-mediated pathway.Exp Dermatol. 2022 Nov;31(11):1729-1740. doi: 10.1111/exd.14651. Epub 2022 Aug 4. Exp Dermatol. 2022. PMID: 35876458 Free PMC article.
-
Mast Cell Phenotypic Heterogeneity Impacts the Interplay with Pathogenic Salmonella Typhimurium Bacteria.Eur J Immunol. 2025 Aug;55(8):e70040. doi: 10.1002/eji.70040. Eur J Immunol. 2025. PMID: 40838737 Free PMC article.
References
-
- Voehringer D. Protective and pathological roles of mast cells and basophils. Nat Rev Immunol 2013; 13: 362–375. - PubMed
-
- Wernersson S, Pejler G. Mast cell granules: armed for battle. Nat Rev 2014; 14: 478–494. - PubMed
-
- Pejler G, Rönnberg E, Waern I, Wernersson S. Mast cell proteases: multifaceted regulators of inflammatory disease. Blood 2010; 115: 4981–4990. - PubMed
-
- Blank U, Rivera J. The ins and outs of IgE-dependent mast-cell exocytosis. Trends Immunol 2004; 25: 266–273. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

