Characterization and correction of the false-discovery rates in resting state connectivity using functional near-infrared spectroscopy
- PMID: 28492852
- PMCID: PMC5424771
- DOI: 10.1117/1.JBO.22.5.055002
Characterization and correction of the false-discovery rates in resting state connectivity using functional near-infrared spectroscopy
Abstract
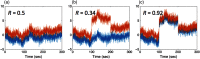
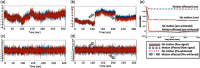
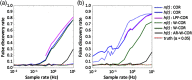
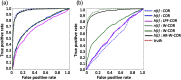
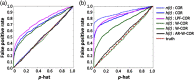
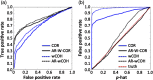
Functional near-infrared spectroscopy (fNIRS) is a noninvasive neuroimaging technique that uses low levels of red to near-infrared light to measure changes in cerebral blood oxygenation. Spontaneous (resting state) functional connectivity (sFC) has become a critical tool for cognitive neuroscience for understanding task-independent neural networks, revealing pertinent details differentiating healthy from disordered brain function, and discovering fluctuations in the synchronization of interacting individuals during hyperscanning paradigms. Two of the main challenges to sFC-NIRS analysis are (i) the slow temporal structure of both systemic physiology and the response of blood vessels, which introduces false spurious correlations, and (ii) motion-related artifacts that result from movement of the fNIRS sensors on the participants’ head and can introduce non-normal and heavy-tailed noise structures. In this work, we systematically examine the false-discovery rates of several time- and frequency-domain metrics of functional connectivity for characterizing sFC-NIRS. Specifically, we detail the modifications to the statistical models of these methods needed to avoid high levels of false-discovery related to these two sources of noise in fNIRS. We compare these analysis procedures using both simulated and experimental resting-state fNIRS data. Our proposed robust correlation method has better performance in terms of being more reliable to the noise outliers due to the motion artifacts.
Figures

References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

