Postmarket Safety Events Among Novel Therapeutics Approved by the US Food and Drug Administration Between 2001 and 2010
- PMID: 28492899
- PMCID: PMC5815036
- DOI: 10.1001/jama.2017.5150
Postmarket Safety Events Among Novel Therapeutics Approved by the US Food and Drug Administration Between 2001 and 2010
Abstract
Importance: Postmarket safety events of novel pharmaceuticals and biologics occur when new safety risks are identified after initial regulatory approval of these therapeutics. These safety events can change how novel therapeutics are used in clinical practice and inform patient and clinician decision making.
Objectives: To characterize the frequency of postmarket safety events among novel therapeutics approved by the US Food and Drug Administration (FDA), and to examine whether any novel therapeutic characteristics known at the time of FDA approval were associated with increased risk.
Design and setting: Cohort study of all novel therapeutics approved by the FDA between January 1, 2001, and December 31, 2010, followed up through February 28, 2017.
Exposures: Novel therapeutic characteristics known at the time of FDA approval, including drug class, therapeutic area, priority review, accelerated approval, orphan status, near-regulatory deadline approval, and regulatory review time.
Main outcomes and measures: A composite of (1) withdrawals due to safety concerns, (2) FDA issuance of incremental boxed warnings added in the postmarket period, and (3) FDA issuance of safety communications.
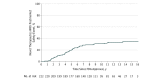
Results: From 2001 through 2010, the FDA approved 222 novel therapeutics (183 pharmaceuticals and 39 biologics). There were 123 new postmarket safety events (3 withdrawals, 61 boxed warnings, and 59 safety communications) during a median follow-up period of 11.7 years (interquartile range [IQR], 8.7-13.8 years), affecting 71 (32.0%) of the novel therapeutics. The median time from approval to first postmarket safety event was 4.2 years (IQR, 2.5-6.0 years), and the proportion of novel therapeutics affected by a postmarket safety event at 10 years was 30.8% (95% CI, 25.1%-37.5%). In multivariable analysis, postmarket safety events were statistically significantly more frequent among biologics (incidence rate ratio [IRR] = 1.93; 95% CI, 1.06-3.52; P = .03), therapeutics indicated for the treatment of psychiatric disease (IRR = 3.78; 95% CI, 1.77-8.06; P < .001), those receiving accelerated approval (IRR = 2.20; 95% CI, 1.15-4.21; P = .02), and those with near-regulatory deadline approval (IRR = 1.90; 95% CI, 1.19-3.05; P = .008); events were statistically significantly less frequent among those with regulatory review times less than 200 days (IRR = 0.46; 95% CI, 0.24-0.87; P = .02).
Conclusions and relevance: Among 222 novel therapeutics approved by the FDA from 2001 through 2010, 32% were affected by a postmarket safety event. Biologics, psychiatric therapeutics, and accelerated and near-regulatory deadline approval were statistically significantly associated with higher rates of events, highlighting the need for continuous monitoring of the safety of novel therapeutics throughout their life cycle.
Conflict of interest statement
Figures



Comment in
-
Characteristics of Novel Therapeutics and Postmarket Safety Events.JAMA. 2017 Sep 19;318(11):1067. doi: 10.1001/jama.2017.11513. JAMA. 2017. PMID: 28975300 No abstract available.
References
-
- Institute of Medicine The Future of Drug Safety: Promoting and Protecting the Health of the Public. Washington, DC: National Academies Press; 2006.
-
- Frank C, Himmelstein DU, Woolhandler S, et al. Era of faster FDA drug approval has also seen increased black-box warnings and market withdrawals. Health Aff (Millwood). 2014;33(8):1453-1459. - PubMed
-
- Carpenter D, Zucker EJ, Avorn J. Drug-review deadlines and safety problems. N Engl J Med. 2008;358(13):1354-1361. - PubMed
-
- US Government Accountability Office Drug Safety: FDA expedites many applications, but data for postapproval oversight need improvement. http://www.gao.gov/products/GAO-16-192. January 14, 2016. Accessed March 10, 2017.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

