Effect of Bevacizumab vs Aflibercept on Visual Acuity Among Patients With Macular Edema Due to Central Retinal Vein Occlusion: The SCORE2 Randomized Clinical Trial
- PMID: 28492910
- PMCID: PMC5710547
- DOI: 10.1001/jama.2017.4568
Effect of Bevacizumab vs Aflibercept on Visual Acuity Among Patients With Macular Edema Due to Central Retinal Vein Occlusion: The SCORE2 Randomized Clinical Trial
Abstract
Importance: Studies have established the efficacy and safety of aflibercept for the treatment of macular edema due to central retinal vein occlusion. Bevacizumab is used off-label to treat this condition despite the absence of supporting data.
Objective: To investigate whether bevacizumab is noninferior to aflibercept for the treatment of macular edema secondary to central retinal or hemiretinal vein occlusion.
Design, setting, and participants: The SCORE2 randomized noninferiority clinical trial was conducted at 66 private practice or academic centers in the United States, and included 362 patients with macular edema due to central retinal or hemiretinal vein occlusion who were randomized 1:1 to receive aflibercept or bevacizumab. The first participant was randomized on September 17, 2014, and the last month 6 visit occurred on May 6, 2016. Analyses included data available as of December 30, 2016.
Interventions: Eyes were randomized to receive intravitreal injection of bevacizumab (1.25 mg; n = 182) or aflibercept (2.0 mg; n = 180) every 4 weeks through month 6.
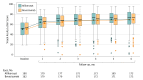
Main outcomes and measures: The primary outcome was mean change in visual acuity (VA) letter score (VALS) from the randomization visit to the 6-month follow-up visit, based on the best-corrected electronic Early Treatment Diabetic Retinopathy Study VALS (scores range from 0-100; higher scores indicate better VA). The noninferiority margin was 5 letters, and statistical testing for noninferiority was based on a 1-sided 97.5% confidence interval.
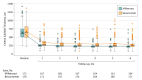
Results: Among 362 randomized participants (mean [SD] age, 69 [12] years; 157 [43.4%] women; mean [SD] VALS at baseline, 50.3 [15.2] [approximate Snellen VA 20/100]), 348 (96.1%) completed the month 6 follow-up visit. At month 6, the mean VALS was 69.3 (a mean increase from baseline of 18.6) in the bevacizumab group and 69.3 (a mean increase from baseline of 18.9) in the aflibercept group (model-based estimate of between-group difference, -0.14; 97.5% CI, -3.07 to ∞; P = .001 for noninferiority), meeting criteria for noninferiority. Ocular adverse events in the aflibercept group included 4 participants with intraocular pressure (IOP) more than 10 mm Hg greater than baseline; ocular adverse events in the bevacizumab group included 1 participant with endophthalmitis (culture negative), 9 with IOP more than 10 mm Hg greater than baseline, 2 with IOP higher than 35 mm Hg, and 1 with angle-closure glaucoma not attributed to the study drug or procedure.
Conclusions and relevance: Among patients with macular edema due to central retinal or hemiretinal vein occlusion, intravitreal bevacizumab was noninferior to aflibercept with respect to visual acuity after 6 months of treatment.
Conflict of interest statement
Figures

Comment in
-
Treatment of Macular Edema Due to Central Retinal Vein Occlusion: Another Score for Repackaged Bevacizumab.JAMA. 2017 May 23;317(20):2067-2069. doi: 10.1001/jama.2017.5899. JAMA. 2017. PMID: 28492939 No abstract available.
-
Gefäßverschlüsse/Antikoagulation.Ophthalmologe. 2018 Aug;115(8):632-633. doi: 10.1007/s00347-018-0749-2. Ophthalmologe. 2018. PMID: 30094661 German. No abstract available.
References
-
- Ponto KA, Elbaz H, Peto T, et al. Prevalence and risk factors of retinal vein occlusion: the Gutenberg Health Study. J Thromb Haemost. 2015;13(7):1254-1263. - PubMed
-
- Mitchell P, Smith W, Chang A. Prevalence and associations of retinal vein occlusion in Australia: the Blue Mountains Eye Study. Arch Ophthalmol. 1996;114(10):1243-1247. - PubMed
-
- Central Vein Occlusion Study Group Natural history and clinical management of central retinal vein occlusion. Arch Ophthalmol. 1997;115(4):486-491. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

