Exploration of Involved Key Genes and Signaling Diversity in Brain Tumors
- PMID: 28493234
- PMCID: PMC11481865
- DOI: 10.1007/s10571-017-0498-9
Exploration of Involved Key Genes and Signaling Diversity in Brain Tumors
Abstract
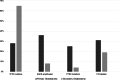
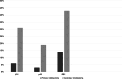
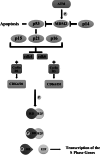
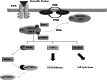
Brain tumors are becoming a major cause of death. The classification of brain tumors has gone through restructuring with regard to some criteria such as the presence or absence of a specific genetic alteration in the 2016 central nervous system World Health Organization update. Two categories of genes with a leading role in tumorigenesis and cancer induction include tumor suppressor genes and oncogenes; tumor suppressor genes are inactivated through a variety of mechanisms that result in their loss of function. As for the oncogenes, overexpression and amplification are the most common mechanisms of alteration. Important cell cycle genes such as p53, ATM, cyclin D2, and Rb have shown altered expression patterns in different brain tumors such as meningioma and astrocytoma. Some genes in signaling pathways have a role in brain tumorigenesis. These pathways include hedgehog, EGFR, Notch, hippo, MAPK, PI3K/Akt, and WNT signaling. It has been shown that telomere length in some brain tumor samples is shortened compared to that in normal cells. As the shortening of telomere length triggers chromosome instability early in brain tumors, it could lead to initiation of cancer. On the other hand, telomerase activity was positive in some brain tumors. It is suggestive that telomere length and telomerase activity are important diagnostic markers in brain tumors. This review focuses on brain tumors with regard to the status of oncogenes, tumor suppressors, cell cycle genes, and genes in signaling pathways as well as the role of telomere length and telomerase in brain tumors.
Keywords: Brain tumors; Cell cycle genes; Oncogenes; Signaling pathways; Telomerase activity; Telomere length; Tumor suppressor genes.
Figures

References
-
- Aldosari N, Bigner SH, Burger PC, Becker L, Kepner JL, Friedman HS, McLendon RE (2002) MYCC and MYCN oncogene amplification in medulloblastoma. A fluorescence in situ hybridization study on paraffin sections from the Children’s Oncology Group. Arch Pathol Lab Med 126:540–544. doi:10.1043/0003-9985(2002)126%3c0540:mamoai%3e2.0.co;2 - PubMed
-
- Alessi DR, Kozlowski MT, Weng Q-P, Morrice N, Avruch J (1998) 3-Phosphoinositide-dependent protein kinase 1 (PDK1) phosphorylates and activates the p70 S6 kinase in vivo and in vitro. Curr Biol 8:69–81 - PubMed
-
- Antonelli M, Buttarelli FR, Giangaspero F (2014) Histopathology and molecular pathology of medulloblastoma. In: The medulloblastoma book. Nova Science Publishers, Inc., New York, p 13–25
-
- Bagchi A, Papazoglu C, Wu Y, Capurso D, Brodt M, Francis D, Bredel M, Vogel H, Mills AA (2007) CHD5 is a tumor suppressor at human 1p36. Cell 128:459–475. doi:10.1016/j.cell.2006.11.052 - PubMed
-
- Bai X, Ma D, Liu A, Shen X, Wang QJ, Liu Y, Jiang Y (2007) Rheb activates mTOR by antagonizing its endogenous inhibitor, FKBP38. Science 318:977–980 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

