Real-time MRI guidance of cardiac interventions
- PMID: 28493526
- PMCID: PMC5675556
- DOI: 10.1002/jmri.25749
Real-time MRI guidance of cardiac interventions
Abstract
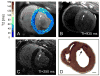
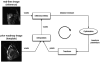
Cardiac magnetic resonance imaging (MRI) is appealing to guide complex cardiac procedures because it is ionizing radiation-free and offers flexible soft-tissue contrast. Interventional cardiac MR promises to improve existing procedures and enable new ones for complex arrhythmias, as well as congenital and structural heart disease. Guiding invasive procedures demands faster image acquisition, reconstruction and analysis, as well as intuitive intraprocedural display of imaging data. Standard cardiac MR techniques such as 3D anatomical imaging, cardiac function and flow, parameter mapping, and late-gadolinium enhancement can be used to gather valuable clinical data at various procedural stages. Rapid intraprocedural image analysis can extract and highlight critical information about interventional targets and outcomes. In some cases, real-time interactive imaging is used to provide a continuous stream of images displayed to interventionalists for dynamic device navigation. Alternatively, devices are navigated relative to a roadmap of major cardiac structures generated through fast segmentation and registration. Interventional devices can be visualized and tracked throughout a procedure with specialized imaging methods. In a clinical setting, advanced imaging must be integrated with other clinical tools and patient data. In order to perform these complex procedures, interventional cardiac MR relies on customized equipment, such as interactive imaging environments, in-room image display, audio communication, hemodynamic monitoring and recording systems, and electroanatomical mapping and ablation systems. Operating in this sophisticated environment requires coordination and planning. This review provides an overview of the imaging technology used in MRI-guided cardiac interventions. Specifically, this review outlines clinical targets, standard image acquisition and analysis tools, and the integration of these tools into clinical workflow.
Level of evidence: 1 Technical Efficacy: Stage 5 J. Magn. Reson. Imaging 2017;46:935-950.
Keywords: cardiac; fast acquisition; image analysis; intervention; real-time.
© 2017 International Society for Magnetic Resonance in Medicine.
Figures






References
-
- Andreassi MG, Ait-Ali L, Botto N, Manfredi S, Mottola G, Picano E. Cardiac catheterization and long-term chromosomal damage in children with congenital heart disease. Eur Heart J. 2006;27:2703–2708. - PubMed
-
- Andreassi MG, Piccaluga E, Guagliumi G, Del Greco M, Gaita F, Picano E. Occupational Health Risks in Cardiac Catheterization Laboratory Workers. Circ Cardiovasc Interv. 2016;9:e003273. - PubMed
-
- Wijnmaalen AP, Van Der Geest RJ, Van Huls Van Taxis CFB, et al. Head-to-head comparison of contrast-enhanced magnetic resonance imaging and electroanatomical voltage mapping to assess post-infarct scar characteristics in patients with ventricular tachycardias: Real-time image integration and reversed registration. Eur Heart J. 2011;32:104–114. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

