Aripiprazole Suppression of Drinking in a Clinical Laboratory Paradigm: Influence of Impulsivity and Self-Control
- PMID: 28493623
- PMCID: PMC5504690
- DOI: 10.1111/acer.13417
Aripiprazole Suppression of Drinking in a Clinical Laboratory Paradigm: Influence of Impulsivity and Self-Control
Abstract
Background: Aspects of impulsivity have been implicated in the development, or maintenance, of alcohol use disorder (AUD). The brain dopamine system is implicated in both reward processing/memory (typically subcortical) and in brain inhibitory control mechanisms (typically cortical). Using a validated clinical laboratory paradigm, the dopamine/serotonin "stabilizing" drug, aripiprazole was evaluated in non-treatment-seeking AUD individuals based on their level of impulsivity/self-control.
Methods: Ninety-nine individuals (77% male; mean age 27; 7.5 drinks per day; 83% heavy drinking days) meeting DSM-IV criteria for alcohol dependence were randomized to aripiprazole (N = 47 evaluable) or placebo (N = 48 evaluable) based on their Barratt Impulsiveness Scale (BIS-11) score (above or below 68). Aripiprazole, or similar placebo, was titrated to 15 mg over 8 days. Drinking was recorded over 6 days under natural conditions. On Day 8, after 1 day of required abstinence, individuals participated in a bar laboratory paradigm that included a priming drink (breath alcohol concentration [BAC] target 0.02 to 0.03 g/dl) and free-choice consumption of up to 8 drinks (max BAC 0.1 g/dl) in exchange for a "bar credit" of $2 per drink (max $16). End points were drinks per day under natural conditions and drinks consumed in the bar laboratory after the priming drink.
Results: There was no significant main effect of aripiprazole or interaction with BIS-11 score during the natural drinking period. However, there was a main effect of aripiprazole on bar laboratory drinking (p = 0.04) and aripiprazole reduced the total number of drinks consumed more among individuals with low self-control (p = 0.034) and increased latency to consume those drinks (p = 0.045) more among those with high impulsivity. Relative to placebo, aripiprazole caused more side effects and increased alcohol-induced sedation, but neither significantly influenced its interaction with impulsivity/self-control scores on drinking.
Conclusions: This paradigm forced a choice between immediate drinking reward and delayed monetary reward. In those with high impulsivity and/or low self-control, aripiprazole shifts the balance away from immediate drinking toward a later reward. Medications targeting cortical dopamine/serotonin balance might show clinical benefit of reduced drinking, among individuals with impulsivity/low self-control.
Keywords: Alcohol; Alcohol Use Disorder; Aripiprazole; Impulsivity; Pharmacotherapy.
Copyright © 2017 by the Research Society on Alcoholism.
Figures

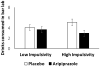

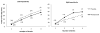
Similar articles
-
Aripiprazole effects on alcohol consumption and subjective reports in a clinical laboratory paradigm--possible influence of self-control.Alcohol Clin Exp Res. 2008 Nov;32(11):1954-61. doi: 10.1111/j.1530-0277.2008.00783.x. Epub 2008 Sep 8. Alcohol Clin Exp Res. 2008. PMID: 18782344 Free PMC article. Clinical Trial.
-
Dopaminergic Genetic Variation Influences Aripiprazole Effects on Alcohol Self-Administration and the Neural Response to Alcohol Cues in a Randomized Trial.Neuropsychopharmacology. 2018 May;43(6):1247-1256. doi: 10.1038/npp.2017.298. Epub 2017 Dec 6. Neuropsychopharmacology. 2018. PMID: 29362512 Free PMC article. Clinical Trial.
-
Aripiprazole for relapse prevention and craving in alcohol use disorder: current evidence and future perspectives.Expert Opin Investig Drugs. 2016 Jun;25(6):719-28. doi: 10.1080/13543784.2016.1175431. Epub 2016 Apr 21. Expert Opin Investig Drugs. 2016. PMID: 27098451 Review.
-
The effect of aripiprazole on cue-induced brain activation and drinking parameters in alcoholics.J Clin Psychopharmacol. 2010 Aug;30(4):365-72. doi: 10.1097/JCP.0b013e3181e75cff. J Clin Psychopharmacol. 2010. PMID: 20571434 Free PMC article. Clinical Trial.
-
Aripiprazole: a drug with a novel mechanism of action and possible efficacy for alcohol dependence.CNS Neurol Disord Drug Targets. 2010 Mar;9(1):50-4. doi: 10.2174/187152710790966731. CNS Neurol Disord Drug Targets. 2010. PMID: 20201815 Review.
Cited by
-
Systematic Review of Combined Pharmacotherapy for the Treatment of Alcohol Use Disorder in Patients Without Comorbid Conditions.CNS Drugs. 2018 Jan;32(1):13-31. doi: 10.1007/s40263-017-0484-2. CNS Drugs. 2018. PMID: 29273901
-
Off-label and investigational drugs in the treatment of alcohol use disorder: A critical review.Front Pharmacol. 2022 Oct 3;13:927703. doi: 10.3389/fphar.2022.927703. eCollection 2022. Front Pharmacol. 2022. PMID: 36263121 Free PMC article. Review.
-
"It's been a long time since I drank like that": A case report of binge drinking associated with aripiprazole.Ment Health Clin. 2024 Jun 3;14(3):212-214. doi: 10.9740/mhc.2024.06.212. eCollection 2024 Jun. Ment Health Clin. 2024. PMID: 38835817 Free PMC article.
-
Prospects for pharmacotherapies to treat alcohol use disorder: an update on recent human studies.Curr Opin Psychiatry. 2019 Jul;32(4):255-265. doi: 10.1097/YCO.0000000000000519. Curr Opin Psychiatry. 2019. PMID: 31107292 Free PMC article. Review.
-
Effects of pharmacological and genetic regulation of COMT activity in alcohol use disorder: a randomized, placebo-controlled trial of tolcapone.Neuropsychopharmacology. 2022 Oct;47(11):1953-1960. doi: 10.1038/s41386-022-01335-z. Epub 2022 May 6. Neuropsychopharmacology. 2022. PMID: 35523943 Free PMC article. Clinical Trial.
References
-
- AMERICAN PSYCHIATRIC ASSOCIATION. Diagnostic and Statistical Manual of Mental Disorders (4th edition): DSM-IV. Washington, DC: American Psychiatric Association; 1994.
-
- ANTON RF, KRANZLER H, BREDER C, MARCUS RN, CARSON WH, HAN J. A randomized, multicenter, double-blind, placebo-controlled study of the efficacy and safety of aripiprazole for the treatment of alcohol dependence. J Clin Psychopharmacol. 2008;28:5–12. - PubMed
-
- ANTON RF, MOAK DH, LATHAM PK. The obsessive compulsive drinking scale: A new method of assessing outcome in alcoholism treatment studies. Arch Gen Psychiatry. 1996;53:225–31. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

