Cetuximab Prevents Methotrexate-Induced Cytotoxicity in Vitro through Epidermal Growth Factor Dependent Regulation of Renal Drug Transporters
- PMID: 28493713
- PMCID: PMC5462489
- DOI: 10.1021/acs.molpharmaceut.7b00308
Cetuximab Prevents Methotrexate-Induced Cytotoxicity in Vitro through Epidermal Growth Factor Dependent Regulation of Renal Drug Transporters
Abstract
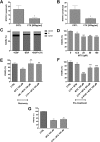
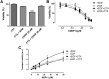
The combination of methotrexate with epidermal growth factor receptor (EGFR) recombinant antibody, cetuximab, is currently being investigated in treatment of head and neck carcinoma. As methotrexate is cleared by renal excretion, we studied the effect of cetuximab on renal methotrexate handling. We used human conditionally immortalized proximal tubule epithelial cells overexpressing either organic anion transporter 1 or 3 (ciPTEC-OAT1/ciPTEC-OAT3) to examine OAT1 and OAT3, and the efflux pumps breast cancer resistance protein (BCRP), multidrug resistance protein 4 (MRP4), and P-glycoprotein (P-gp) in methotrexate handling upon EGF or cetuximab treatment. Protein kinase microarrays and knowledge-based pathway analysis were used to predict EGFR-mediated transporter regulation. Cytotoxic effects of methotrexate were evaluated using the dimethylthiazol bromide (MTT) viability assay. Methotrexate inhibited OAT-mediated fluorescein uptake and decreased efflux of Hoechst33342 and glutathione-methylfluorescein (GS-MF), which suggested involvement of OAT1/3, BCRP, and MRP4 in transepithelial transport, respectively. Cetuximab reversed the EGF-increased expression of OAT1 and BCRP as well as their membrane expressions and transport activities, while MRP4 and P-gp were increased. Pathway analysis predicted cetuximab-induced modulation of PKC and PI3K pathways downstream EGFR/ERBB2/PLCg. Pharmacological inhibition of ERK decreased expression of OAT1 and BCRP, while P-gp and MRP4 were increased. AKT inhibition reduced all transporters. Exposure to methotrexate for 24 h led to a decreased viability, an effect that was reversed by cetuximab. In conclusion, cetuximab downregulates OAT1 and BCRP while upregulating P-gp and MRP4 through an EGFR-mediated regulation of PI3K-AKT and MAPKK-ERK pathways. Consequently, cetuximab attenuates methotrexate-induced cytotoxicity, which opens possibilities for further research into nephroprotective comedication therapies.
Keywords: combination therapy; drug disposition; drug transporters; kinase signaling; renal proximal tubule.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Bright R. D. Methotrexate in the treatment of psoriasis. Cutis. 1999, 64 (5), 332–334. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

