Shear-Wave Elastography: Basic Physics and Musculoskeletal Applications
- PMID: 28493799
- PMCID: PMC5452887
- DOI: 10.1148/rg.2017160116
Shear-Wave Elastography: Basic Physics and Musculoskeletal Applications
Abstract
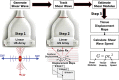
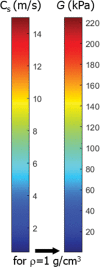
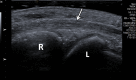
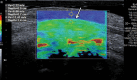
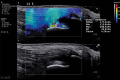
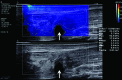
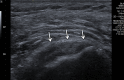
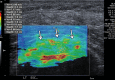
In the past 2 decades, sonoelastography has been progressively used as a tool to help evaluate soft-tissue elasticity and add to information obtained with conventional gray-scale and Doppler ultrasonographic techniques. Recently introduced on clinical scanners, shear-wave elastography (SWE) is considered to be more objective, quantitative, and reproducible than compression sonoelastography with increasing applications to the musculoskeletal system. SWE uses an acoustic radiation force pulse sequence to generate shear waves, which propagate perpendicular to the ultrasound beam, causing transient displacements. The distribution of shear-wave velocities at each pixel is directly related to the shear modulus, an absolute measure of the tissue's elastic properties. Shear-wave images are automatically coregistered with standard B-mode images to provide quantitative color elastograms with anatomic specificity. Shear waves propagate faster through stiffer contracted tissue, as well as along the long axis of tendon and muscle. SWE has a promising role in determining the severity of disease and treatment follow-up of various musculoskeletal tissues including tendons, muscles, nerves, and ligaments. This article describes the basic ultrasound physics of SWE and its applications in the evaluation of various traumatic and pathologic conditions of the musculoskeletal system. ©RSNA, 2017.
Figures
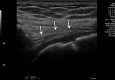
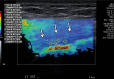
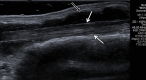
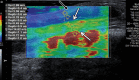
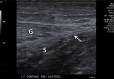


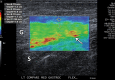
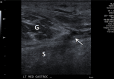
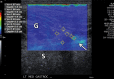
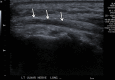
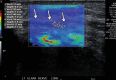
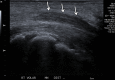
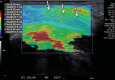
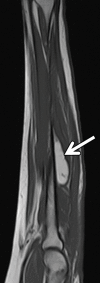
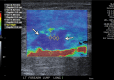
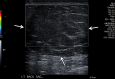
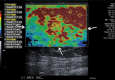
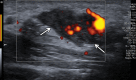
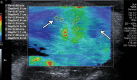
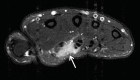
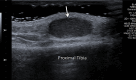
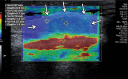
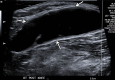
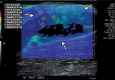
References
-
- Jacobson JA. Musculoskeletal ultrasound: focused impact on MRI. AJR Am J Roentgenol 2009;193(3):619–627. - PubMed
-
- Klauser AS, Miyamoto H, Bellmann-Weiler R, Feuchtner GM, Wick MC, Jaschke WR. Sonoelastography: musculoskeletal applications. Radiology 2014;272(3):622–633. - PubMed
-
- Arda K, Ciledag N, Aktas E, Aribas BK, Köse K. Quantitative assessment of normal soft-tissue elasticity using shear-wave ultrasound elastography. AJR Am J Roentgenol 2011;197(3):532–536. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

