Functional male accessory glands and fertility in Drosophila require novel ecdysone receptor
- PMID: 28493870
- PMCID: PMC5444863
- DOI: 10.1371/journal.pgen.1006788
Functional male accessory glands and fertility in Drosophila require novel ecdysone receptor
Erratum in
-
Correction: Functional male accessory glands and fertility in Drosophila require novel ecdysone receptor.PLoS Genet. 2017 Jul 12;13(7):e1006893. doi: 10.1371/journal.pgen.1006893. eCollection 2017 Jul. PLoS Genet. 2017. PMID: 28700620 Free PMC article.
-
Correction: Functional male accessory glands and fertility in Drosophila require novel ecdysone receptor.PLoS Genet. 2018 Jul 13;14(7):e1007524. doi: 10.1371/journal.pgen.1007524. eCollection 2018 Jul. PLoS Genet. 2018. PMID: 30005076 Free PMC article.
Abstract
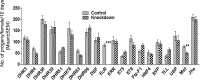
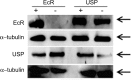
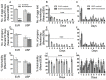
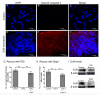
In many insects, the accessory gland, a secretory tissue of the male reproductive system, is essential for male fertility. Male accessory gland is the major source of proteinaceous secretions, collectively called as seminal proteins (or accessory gland proteins), which upon transfer, manipulate the physiology and behavior of mated females. Insect hormones such as ecdysteroids and juvenoids play a key role in accessory gland development and protein synthesis but little is known about underlying molecular players and their mechanism of action. Therefore, in the present study, we examined the roles of hormone-dependent transcription factors (Nuclear Receptors), in accessory gland development, function and male fertility of a genetically tractable insect model, Drosophila melanogaster. First, we carried out an RNAi screen involving 19 hormone receptors, individually and specifically, in a male reproductive tissue (accessory gland) for their requirement in Drosophila male fertility. Subsequently, by using independent RNAi/ dominant negative forms, we show that Ecdysone Receptor (EcR) is essential for male fertility due to its requirement in the normal development of accessory glands in Drosophila: EcR depleted glands fail to make seminal proteins and have dying cells. Further, our data point to a novel ecdysone receptor that does not include Ultraspiracle but is probably comprised of EcR isoforms in Drosophila male accessory glands. Our data suggest that this novel ecdysone receptor might act downstream of homeodomain transcription factor paired (prd) in the male accessory gland. Overall, the study suggests novel ecdysone receptor as an important player in the hormonal regulation of seminal protein production and insect male fertility.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





Similar articles
-
Regulation of male fertility and accessory gland gene expression by the Drosophila HR39 nuclear receptor.Dev Biol. 2021 Nov;479:51-60. doi: 10.1016/j.ydbio.2021.07.011. Epub 2021 Jul 29. Dev Biol. 2021. PMID: 34331899 Free PMC article.
-
Fecundity is optimized by levels of nutrient signal-dependent expression of Dve and EcR in Drosophila male accessory gland.Dev Biol. 2024 Apr;508:8-23. doi: 10.1016/j.ydbio.2024.01.004. Epub 2024 Jan 8. Dev Biol. 2024. PMID: 38199580
-
Mating induces switch from hormone-dependent to hormone-independent steroid receptor-mediated growth in Drosophila secondary cells.PLoS Biol. 2019 Oct 7;17(10):e3000145. doi: 10.1371/journal.pbio.3000145. eCollection 2019 Oct. PLoS Biol. 2019. PMID: 31589603 Free PMC article.
-
Arthropod nuclear receptors and their role in molting.FEBS J. 2009 Nov;276(21):6128-57. doi: 10.1111/j.1742-4658.2009.07347.x. Epub 2009 Sep 30. FEBS J. 2009. PMID: 19796154 Review.
-
Nuclear receptors linking physiology and germline stem cells in Drosophila.Vitam Horm. 2021;116:327-362. doi: 10.1016/bs.vh.2020.12.008. Vitam Horm. 2021. PMID: 33752824 Free PMC article. Review.
Cited by
-
Divergent allocation of sperm and the seminal proteome along a competition gradient in Drosophila melanogaster.Proc Natl Acad Sci U S A. 2019 Sep 3;116(36):17925-17933. doi: 10.1073/pnas.1906149116. Epub 2019 Aug 20. Proc Natl Acad Sci U S A. 2019. PMID: 31431535 Free PMC article.
-
Correction: Functional male accessory glands and fertility in Drosophila require novel ecdysone receptor.PLoS Genet. 2018 Jul 13;14(7):e1007524. doi: 10.1371/journal.pgen.1007524. eCollection 2018 Jul. PLoS Genet. 2018. PMID: 30005076 Free PMC article.
-
The Drosophila seminal proteome and its role in postcopulatory sexual selection.Philos Trans R Soc Lond B Biol Sci. 2020 Dec 7;375(1813):20200072. doi: 10.1098/rstb.2020.0072. Epub 2020 Oct 19. Philos Trans R Soc Lond B Biol Sci. 2020. PMID: 33070726 Free PMC article. Review.
-
Anopheles coluzzii stearoyl-CoA desaturase is essential for adult female survival and reproduction upon blood feeding.PLoS Pathog. 2021 May 20;17(5):e1009486. doi: 10.1371/journal.ppat.1009486. eCollection 2021 May. PLoS Pathog. 2021. PMID: 34015060 Free PMC article.
-
Correction: Functional male accessory glands and fertility in Drosophila require novel ecdysone receptor.PLoS Genet. 2017 Jul 12;13(7):e1006893. doi: 10.1371/journal.pgen.1006893. eCollection 2017 Jul. PLoS Genet. 2017. PMID: 28700620 Free PMC article.
References
-
- Avila FW, Sirot LK, LaFlamme BA, Rubinstein CD, Wolfner MF (2011) Insect seminal fluid proteins: identification and function. Annu Rev Entomol 56: 21–40. doi: 10.1146/annurev-ento-120709-144823 - DOI - PMC - PubMed
-
- Sirot LK, LaFlamme BA, Sitnik JL, Rubinstein CD, Avila FW, et al. (2009) Molecular social interactions: Drosophila melanogaster seminal fluid proteins as a case study. Adv Genet 68: 23–56. doi: 10.1016/S0065-2660(09)68002-0 - DOI - PMC - PubMed
-
- Chapman T, Bangham J, Vinti G, Seifried B, Lung O, et al. (2003) The sex peptide of Drosophila melanogaster: female post-mating responses analyzed by using RNA interference. Proc Natl Acad Sci U S A 100: 9923–9928. doi: 10.1073/pnas.1631635100 - DOI - PMC - PubMed
-
- Liu H, Kubli E (2003) Sex-peptide is the molecular basis of the sperm effect in Drosophila melanogaster. Proc Natl Acad Sci U S A 100: 9929–9933. doi: 10.1073/pnas.1631700100 - DOI - PMC - PubMed
-
- Ravi Ram K, Wolfner MF (2007a) Seminal influences: Drosophila Acps and the molecular interplay between males and females during reproduction. Integr Comp Biol 47: 427–445. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

