CO2 driven endotracheal tube cuff control in critically ill patients: A randomized controlled study
- PMID: 28493877
- PMCID: PMC5426597
- DOI: 10.1371/journal.pone.0175476
CO2 driven endotracheal tube cuff control in critically ill patients: A randomized controlled study
Abstract
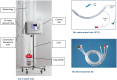
Background: To determine the safety and clinical efficacy of an innovative integrated airway system (AnapnoGuard™ 100 system) that continuously monitors and controls the cuff pressure (Pcuff), while facilitating the aspiration of subglottic secretions (SS).
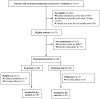
Methods: This was a prospective, single centre, open-label, randomized, controlled feasibility and safety trial. The primary endpoint of the study was the rate of device related adverse events (AE) and serious AE (SAE) as a result of using AnapnoGuard (AG) 100 during mechanical ventilation. Secondary endpoints were: (1) mechanical complications rate (2) ICU staff satisfaction; (3) VAP occurrence; (4) length of mechanical ventilation; (5) length of Intensive Care Unit stay and mortality; (6) volume of evacuated subglottic secretions. Sixty patients were randomized to be intubated with the AG endotracheal-tube (ETT) and connected to the AG 100 system allowing Pcuff adjustment and SS aspiration; or with an ETT combined with SS drainage and Pcuff controlled manually.
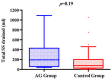
Results: No difference in adverse events rate was identified between the groups. The use of AG system was associated with a significantly higher incidence of Pcuff determinations in the safety range (97.3% vs. 71%; p<0.01) and a trend to a greater volume of aspirated SS secretions: (192.0[64-413] ml vs. 150[50-200], p = 0.19 (total)); (57.8[20-88.7] ml vs. 50[18.7-62] ml, p = 0.11 (daily)). No inter-group difference was detected using AG system vs. controls in terms of post-extubation throat pain level (0 [0-2] vs. 0 [0-3]; p = 0.7), hoarseness (42.9% vs. 75%; p = 0.55) and tracheal mucosa oedema (16.7% vs. 10%; p = 0.65). Patients enrolled in the AG group had a trend to reduced VAP risk of ventilator-associated pneumonia(VAP) (14.8% vs. 40%; p = 0.06), which were more frequently monomicrobial (25% vs. 70%; p = 0.03). No statistically significant difference was observed in duration of mechanical ventilation, ICU stay, and mortality.
Conclusions: The use AG 100 system and AG tube in critically ill intubated patients is safe and effective in Pcuff control and SS drainage. Its protective role against VAP needs to be confirmed in a larger randomized trial.
Trial registration: ClinicalTrials.gov NCT01550978. Date of registration: February 21, 2012.
Conflict of interest statement
Figures




References
-
- Stauffer JL. Complications of translaryngeal intubation In: McGraw-Hill IE, editor. Principles and Practice of Mechanical Ventilation. First Edition ed. New York: MJ Tobin; 1994. p. 711–48.
-
- Estes RJ, Meduri GU. The pathogenesis of ventilator-associated pneumonia: I. Mechanisms of bacterial transcolonization and airway inoculation. Intensive care medicine. 1995;21(4):365–83. Epub 1995/04/01. - PubMed
-
- Craven DE. Nosocomial pneumonia: new concepts on an old disease. Infection control and hospital epidemiology. 1988;9(2):57–8. Epub 1988/02/01. - PubMed
-
- Nseir S, Brisson H, Marquette CH, Chaud P, Di Pompeo C, Diarra M, et al. Variations in endotracheal cuff pressure in intubated critically ill patients: prevalence and risk factors. European journal of anaesthesiology. 2009;26(3):229–34. Epub 2009/02/28. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

