The host ubiquitin-dependent segregase VCP/p97 is required for the onset of human cytomegalovirus replication
- PMID: 28494016
- PMCID: PMC5426786
- DOI: 10.1371/journal.ppat.1006329
The host ubiquitin-dependent segregase VCP/p97 is required for the onset of human cytomegalovirus replication
Abstract
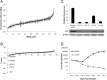
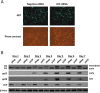
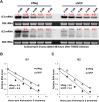
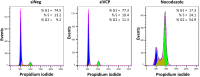
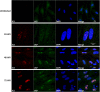
The human cytomegalovirus major immediate early proteins IE1 and IE2 are critical drivers of virus replication and are considered pivotal in determining the balance between productive and latent infection. IE1 and IE2 are derived from the same primary transcript by alternative splicing and regulation of their expression likely involves a complex interplay between cellular and viral factors. Here we show that knockdown of the host ubiquitin-dependent segregase VCP/p97, results in loss of IE2 expression, subsequent suppression of early and late gene expression and, ultimately, failure in virus replication. RNAseq analysis showed increased levels of IE1 splicing, with a corresponding decrease in IE2 splicing following VCP knockdown. Global analysis of viral transcription showed the expression of a subset of viral genes is not reduced despite the loss of IE2 expression, including UL112/113. Furthermore, Immunofluorescence studies demonstrated that VCP strongly colocalised with the viral replication compartments in the nucleus. Finally, we show that NMS-873, a small molecule inhibitor of VCP, is a potent HCMV antiviral with potential as a novel host targeting therapeutic for HCMV infection.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
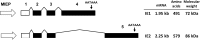



Similar articles
-
The Human Cytomegalovirus Transmembrane Protein pUL50 Induces Loss of VCP/p97 and Is Regulated by a Small Isoform of pUL50.J Virol. 2020 Jun 16;94(13):e00110-20. doi: 10.1128/JVI.00110-20. Print 2020 Jun 16. J Virol. 2020. PMID: 32321808 Free PMC article.
-
The human cytomegalovirus IE2 and UL112-113 proteins accumulate in viral DNA replication compartments that initiate from the periphery of promyelocytic leukemia protein-associated nuclear bodies (PODs or ND10).J Virol. 1999 Dec;73(12):10458-71. doi: 10.1128/JVI.73.12.10458-10471.1999. J Virol. 1999. PMID: 10559364 Free PMC article.
-
Disruption of PML-associated nuclear bodies by IE1 correlates with efficient early stages of viral gene expression and DNA replication in human cytomegalovirus infection.Virology. 2000 Aug 15;274(1):39-55. doi: 10.1006/viro.2000.0448. Virology. 2000. PMID: 10936087
-
Bright and Early: Inhibiting Human Cytomegalovirus by Targeting Major Immediate-Early Gene Expression or Protein Function.Viruses. 2020 Jan 16;12(1):110. doi: 10.3390/v12010110. Viruses. 2020. PMID: 31963209 Free PMC article. Review.
-
Strategic role of the ubiquitin-dependent segregase p97 (VCP or Cdc48) in DNA replication.Chromosoma. 2017 Feb;126(1):17-32. doi: 10.1007/s00412-016-0587-4. Epub 2016 Apr 18. Chromosoma. 2017. PMID: 27086594 Review.
Cited by
-
Identification of lead anti-human cytomegalovirus compounds targeting MAP4K4 via machine learning analysis of kinase inhibitor screening data.PLoS One. 2018 Jul 26;13(7):e0201321. doi: 10.1371/journal.pone.0201321. eCollection 2018. PLoS One. 2018. PMID: 30048526 Free PMC article.
-
The Human Cytomegalovirus Transmembrane Protein pUL50 Induces Loss of VCP/p97 and Is Regulated by a Small Isoform of pUL50.J Virol. 2020 Jun 16;94(13):e00110-20. doi: 10.1128/JVI.00110-20. Print 2020 Jun 16. J Virol. 2020. PMID: 32321808 Free PMC article.
-
The p97 Inhibitor UPCDC-30245 Blocks Endo-Lysosomal Degradation.Pharmaceuticals (Basel). 2022 Feb 7;15(2):204. doi: 10.3390/ph15020204. Pharmaceuticals (Basel). 2022. PMID: 35215314 Free PMC article.
-
Asparagine Deprivation Causes a Reversible Inhibition of Human Cytomegalovirus Acute Virus Replication.mBio. 2019 Oct 8;10(5):e01651-19. doi: 10.1128/mBio.01651-19. mBio. 2019. PMID: 31594813 Free PMC article.
-
Control of Immediate Early Gene Expression for Human Cytomegalovirus Reactivation.Front Cell Infect Microbiol. 2020 Sep 17;10:476. doi: 10.3389/fcimb.2020.00476. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 33072616 Free PMC article. Review.
References
-
- Nelson JA, Gnann JW Jr., Ghazal P (1990) Regulation and tissue-specific expression of human cytomegalovirus. Curr Top Microbiol Immunol 154: 75–100. - PubMed
-
- Boehler A, Schaffner A, Salomon F, Keusch G (1994) Cytomegalovirus disease of late onset following renal transplantation: a potentially fatal entity. Scand J Infect Dis 26: 369–373. - PubMed
-
- Ballard RA, Drew WL, Hufnagle KG, Riedel PA (1979) Acquired cytomegalovirus infection in preterm infants. Am J Dis Child 133: 482–485. - PubMed
-
- Adler SP (1983) Transfusion-associated cytomegalovirus infections. Rev Infect Dis 5: 977–993. - PubMed
-
- Einhorn L, Ost A (1984) Cytomegalovirus infection of human blood cells. J Infect Dis 149: 207–214. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

