Pannexin 3 regulates proliferation and differentiation of odontoblasts via its hemichannel activities
- PMID: 28494020
- PMCID: PMC5426780
- DOI: 10.1371/journal.pone.0177557
Pannexin 3 regulates proliferation and differentiation of odontoblasts via its hemichannel activities
Abstract
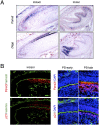
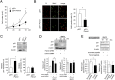
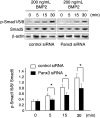
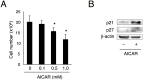
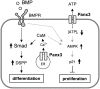
Highly coordinated regulation of cell proliferation and differentiation contributes to the formation of functionally shaped and sized teeth; however, the mechanism underlying the switch from cell cycle exit to cell differentiation during odontogenesis is poorly understood. Recently, we identified pannexin 3 (Panx3) as a member of the pannexin gap junction protein family from tooth germs. The expression of Panx3 was predominately localized in preodontoblasts that arise from dental papilla cells and can differentiate into dentin-secreting odontoblasts. Panx3 also co-localized with p21, a cyclin-dependent kinase inhibitor protein, in preodontoblasts. Panx3 was expressed in primary dental mesenchymal cells and in the mDP dental mesenchymal cell line. Both Panx3 and p21 were induced during the differentiation of mDP cells. Overexpression of Panx3 in mDP cells reduced cell proliferation via up-regulation of p21, but not of p27, and promoted the Bone morphogenetic protein 2 (BMP2)-induced phosphorylation of Smad1/5/8 and the expression of dentin sialophosphoprotein (Dspp), a marker of differentiated odontoblasts. Furthermore, Panx3 released intracellular ATP into the extracellular space through its hemichannel and induced the phosphorylation of AMP-activated protein kinase (AMPK). 5-Aminoimidazole-4-carboxamide-ribonucleoside (AICAR), an activator of AMPK, reduced mDP cell proliferation and induced p21 expression. Conversely, knockdown of endogenous Panx3 by siRNA inhibited AMPK phosphorylation, p21 expression, and the phosphorylation of Smad1/5/8 even in the presence of BMP2. Taken together, our results suggest that Panx3 modulates intracellular ATP levels, resulting in the inhibition of odontoblast proliferation through the AMPK/p21 signaling pathway and promotion of cell differentiation by the BMP/Smad signaling pathway.
Conflict of interest statement
Figures





Similar articles
-
Pannexin 3 channels in health and disease.Purinergic Signal. 2021 Dec;17(4):577-589. doi: 10.1007/s11302-021-09805-7. Epub 2021 Jul 12. Purinergic Signal. 2021. PMID: 34250568 Free PMC article. Review.
-
Bone morphogenetic protein 2 mediates dentin sialophosphoprotein expression and odontoblast differentiation via NF-Y signaling.J Biol Chem. 2008 Jul 11;283(28):19359-70. doi: 10.1074/jbc.M709492200. Epub 2008 Apr 18. J Biol Chem. 2008. PMID: 18424784 Free PMC article.
-
Prrx2, the paired-related homeobox transcription factor, functions as a potential regulator of pannexin 3 expression in odontoblast differentiation.J Oral Biosci. 2025 Mar;67(1):100601. doi: 10.1016/j.job.2024.100601. Epub 2024 Dec 27. J Oral Biosci. 2025. PMID: 39733924
-
Expression of Pannexin3 in human odontoblast-like cells and its hemichannel function in mediating ATP release.Arch Oral Biol. 2015 Oct;60(10):1510-6. doi: 10.1016/j.archoralbio.2015.07.005. Epub 2015 Jul 13. Arch Oral Biol. 2015. PMID: 26263540
-
The Role of Pannexin 3 in Bone Biology.J Dent Res. 2017 Apr;96(4):372-379. doi: 10.1177/0022034516678203. Epub 2016 Nov 13. J Dent Res. 2017. PMID: 27837015 Free PMC article. Review.
Cited by
-
Unraveling the Role of the Apical Papilla During Dental Root Maturation.Front Cell Dev Biol. 2021 May 6;9:665600. doi: 10.3389/fcell.2021.665600. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34026757 Free PMC article. Review.
-
ATP transporters in the joints.Purinergic Signal. 2021 Dec;17(4):591-605. doi: 10.1007/s11302-021-09810-w. Epub 2021 Aug 15. Purinergic Signal. 2021. PMID: 34392490 Free PMC article. Review.
-
Gelatinases Cleave Dentin Sialoprotein Intracellularly.Front Physiol. 2020 Jun 25;11:686. doi: 10.3389/fphys.2020.00686. eCollection 2020. Front Physiol. 2020. PMID: 32670089 Free PMC article.
-
The functional effects of Piezo channels in mesenchymal stem cells.Stem Cell Res Ther. 2023 Aug 26;14(1):222. doi: 10.1186/s13287-023-03452-y. Stem Cell Res Ther. 2023. PMID: 37633928 Free PMC article. Review.
-
Pannexin 3 channels in health and disease.Purinergic Signal. 2021 Dec;17(4):577-589. doi: 10.1007/s11302-021-09805-7. Epub 2021 Jul 12. Purinergic Signal. 2021. PMID: 34250568 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

