Association of Minimal Residual Disease With Clinical Outcome in Pediatric and Adult Acute Lymphoblastic Leukemia: A Meta-analysis
- PMID: 28494052
- PMCID: PMC5824235
- DOI: 10.1001/jamaoncol.2017.0580
Association of Minimal Residual Disease With Clinical Outcome in Pediatric and Adult Acute Lymphoblastic Leukemia: A Meta-analysis
Abstract
Importance: Minimal residual disease (MRD) refers to the presence of disease in cases deemed to be in complete remission by conventional pathologic analysis. Assessing the association of MRD status following induction therapy in patients with acute lymphoblastic leukemia (ALL) with relapse and mortality may improve the efficiency of clinical trials and accelerate drug development.
Objective: To quantify the relationships between event-free survival (EFS) and overall survival (OS) with MRD status in pediatric and adult ALL using publications of clinical trials and other databases.
Data sources: Clinical studies in ALL identified via searches of PubMed, MEDLINE, and clinicaltrials.gov.
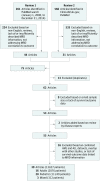
Study selection: Our search and study screening process adhered to the PRISMA Guidelines. Studies that addressed EFS or OS by MRD status in patients with ALL were included; reviews, abstracts, and studies with fewer than 30 patients or insufficient MRD description were excluded.
Data extraction and synthesis: Study sample size, patient age, follow-up time, timing of MRD assessment (postinduction or consolidation), MRD detection method, phenotype/genotype (B cell, T cell, Philadelphia chromosome), and EFS and OS. Searches of PubMed and MEDLINE identified 566 articles. A parallel search on clinicaltrials.gov found 67 closed trials and 62 open trials as of 2014. Merging results of 2 independent searches and applying exclusions gave 39 publications in 3 arms of patient populations (adult, pediatric, and mixed). We performed separate meta-analyses for each of these 3 subpopulations.
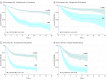
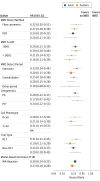
Results: The 39 publications comprised 13 637 patients: 16 adult studies (2076 patients), 20 pediatric (11 249 patients), and 3 mixed (312 patients). The EFS hazard ratio (HR) for achieving MRD negativity is 0.23 (95% Bayesian credible interval [BCI] 0.18-0.28) for pediatric patients and 0.28 (95% BCI, 0.24-0.33) for adults. The respective HRs in OS are 0.28 (95% BCI, 0.19-0.41) and 0.28 (95% BCI, 0.20-0.39). The effect was similar across all subgroups and covariates.
Conclusions and relevance: The value of having achieved MRD negativity is substantial in both pediatric and adult patients with ALL. These results are consistent across therapies, methods of and times of MRD assessment, cutoff levels, and disease subtypes. Minimal residual disease status warrants consideration as an early measure of disease response for evaluating new therapies, improving the efficiency of clinical trials, accelerating drug development, and for regulatory approval. A caveat is that an accelerated approval of a particular new drug using an intermediate end point, such as MRD, would require confirmation using traditional efficacy end points.
Conflict of interest statement
Figures

References
-
- Kantarjian H, Shah NP, Hochhaus A, et al. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2010;362(24):2260-2270. - PubMed
-
- Saglio G, Kim DW, Issaragrisil S, et al. ; ENESTnd Investigators . Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med. 2010;362(24):2251-2259. - PubMed
-
- Buccisano F, Maurillo L, Gattei V, et al. The kinetics of reduction of minimal residual disease impacts on duration of response and survival of patients with acute myeloid leukemia. Leukemia. 2006;20(10):1783-1789. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

