Cooperating Commensals Restore Colonization Resistance to Vancomycin-Resistant Enterococcus faecium
- PMID: 28494240
- PMCID: PMC5494988
- DOI: 10.1016/j.chom.2017.04.002
Cooperating Commensals Restore Colonization Resistance to Vancomycin-Resistant Enterococcus faecium
Abstract
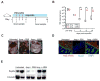
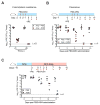
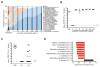
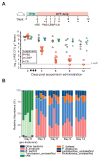
Antibiotic-mediated microbiota destruction and the consequent loss of colonization resistance can result in intestinal domination with vancomycin-resistant Enterococcus (VRE), leading to bloodstream infection in hospitalized patients. Clearance of VRE remains a challenging goal that, if achieved, would reduce systemic VRE infections and patient-to-patient transmission. Although obligate anaerobic commensal bacteria have been associated with colonization resistance to VRE, the specific bacterial species involved remain undefined. Herein, we demonstrate that a precisely defined consortium of commensal bacteria containing the Clostridium cluster XIVa species Blautia producta and Clostridium bolteae restores colonization resistance against VRE and clears VRE from the intestines of mice. While C. bolteae did not directly mediate VRE clearance, it enabled intestinal colonization with B. producta, which directly inhibited VRE growth. These findings suggest that therapeutic or prophylactic administration of defined bacterial consortia to individuals with compromised microbiota composition may reduce inter-patient transmission and intra-patient dissemination of highly antibiotic-resistant pathogens.
Keywords: Blautia product; Enterococcus; Enterococcus faecium; antibiotic resistance; colonization resistance; commensal bacteria; intestinal domination; microbiome; microbiota; vancomycin resistance.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures


References
-
- Antibiotic Resistance Threats in the United States. 2013 - PubMed
-
- Arias CA, Murray BE. Emergence and management of drug-resistant enterococcal infections. Expert Rev Anti Infect Ther. 2008;6:637–655. - PubMed
-
- Atarashi K, Tanoue T, Oshima K, Suda W, Nagano Y, Nishikawa H, Fukuda S, Saito T, Narushima S, Hase K, et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013;500:232–236. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

