A comparative study of sorafenib and metronomic chemotherapy for Barcelona Clinic Liver Cancer-stage C hepatocellular carcinoma with poor liver function
- PMID: 28494528
- PMCID: PMC5497665
- DOI: 10.3350/cmh.2016.0071
A comparative study of sorafenib and metronomic chemotherapy for Barcelona Clinic Liver Cancer-stage C hepatocellular carcinoma with poor liver function
Abstract
Background/aims: Metronomic chemotherapy (MET) is frequently administered in comparatively low doses as a continuous chemotherapeutic agent. The aim of this study was to evaluate the feasibility and overall survival (OS) of MET compared to sorafenib for advanced hepatocellular carcinoma (HCC) patients with portal vein tumor thrombosis (PVTT).
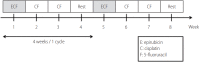
Methods: A total of 54 patients with advanced HCC and PVTT who had undergone MET were analyzed between 2005 and 2013. A total of 53 patients who had undergone sorafenib therapy were analyzed as the control group. The primary endpoint of this study was OS.
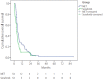
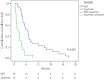
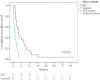
Results: The median number of MET cycles was two (1-15). The OS values for the MET group and sorafenib group were 158 days (132-184) and 117 days (92-142), respectively (P=0.029). The Cox proportional-hazard model showed that a higher risk of death was correlated with higher serum alpha fetoprotein level (≥400 mg/dL, hazard ratio [HR]=1.680, P=0.014) and Child-Pugh class B (HR=1.856, P=0.008).
Conclusions: MET was associated with more favorable outcomes in terms of overall survival than was sorafenib in patients with advanced HCC with PVTT, especially in patients with poor liver function. Therefore, MET can be considered as a treatment option in patients with advanced HCC with PVTT and poor liver function.
Keywords: Administration, Metronomic; Carcinoma, Hepatocellular; Portal vein; Sorafenib; Thrombosis.
Conflict of interest statement
Figures

Comment in
-
Can metronomic chemotherapy be an alternative to sorafenib in advanced hepatocellular carcinoma?Clin Mol Hepatol. 2017 Jun;23(2):123-124. doi: 10.3350/cmh.2017.0107. Epub 2017 Jun 23. Clin Mol Hepatol. 2017. PMID: 28669140 Free PMC article. No abstract available.
References
-
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. - PubMed
-
- Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. - PubMed
-
- Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329–338. - PubMed
-
- Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–1917. - PubMed
-
- Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

