Ferroptosis: A Novel Anti-tumor Action for Cisplatin
- PMID: 28494534
- PMCID: PMC5912137
- DOI: 10.4143/crt.2016.572
Ferroptosis: A Novel Anti-tumor Action for Cisplatin
Abstract
Purpose: Ferroptosis is a new mode of regulated cell death, which is completely distinct from other cell death modes based on morphological, biochemical, and genetic criteria. This study evaluated the therapeutic role of ferroptosis in classic chemotherapy drugs, including the underlying mechanism.
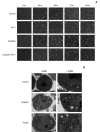
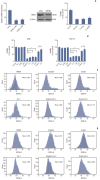
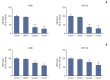
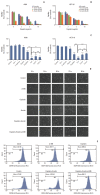
Materials and methods: Cell viabilitywas detected by using the methylthiazoltetrazlium dye uptake method. RNAiwas used to knockout iron-responsive element binding protein 2, and polymerase chain reaction, western blot was used to evaluate the efficiency. Intracellular reduced glutathione level and glutathione peroxidases activitywere determined by related assay kit. Intracellularreactive oxygen species levelswere determined by flowcytometry. Electron microscopywas used to observe ultrastructure changes in cell.
Results: Among five chemotherapeutic drugs screened in this study, cisplatin was found to be an inducer for both ferroptosis and apoptosis in A549 and HCT116 cells. The depletion of reduced glutathione caused by cisplatin and the inactivation of glutathione peroxidase played the vital role in the underlying mechanism. Besides, combination therapy of cisplatin and erastin showed significant synergistic effect on their anti-tumor activity.
Conclusion: Ferroptosis had great potential to become a new approach in anti-tumor therapies and make up for some classic drugs, which open up a new way for their utility in clinic.
Keywords: Cisplatin; Erastin; Ferroptosis; Glutathione; Glutathione peroxidase.
Conflict of interest statement
Conflict of interest relevant to this article was not reported.
Figures

References
-
- Dolma S, Lessnick SL, Hahn WC, Stockwell BR. Identification of genotype-selective antitumor agents using synthetic lethal chemical screening in engineered human tumor cells. Cancer Cell. 2003;3:285–96. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

