Globule Leukocytes and Other Mast Cells in the Mouse Intestine
- PMID: 28494703
- PMCID: PMC7732699
- DOI: 10.1177/0300985817705174
Globule Leukocytes and Other Mast Cells in the Mouse Intestine
Abstract
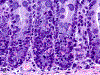
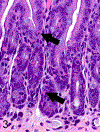
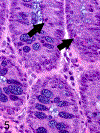
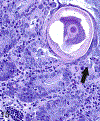
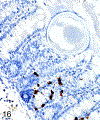
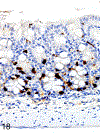
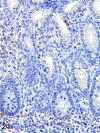
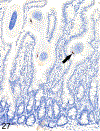
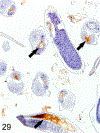
Only 2 major mast cell (MC) subtypes are commonly recognized in the mouse: the large connective tissue mast cells (CTMCs) and the mucosal mast cells (MMCs). Interepithelial mucosal inflammatory cells, most commonly identified as globule leukocytes (GLs), represent a third MC subtype in mice, which we term interepithelial mucosal mast cells (ieMMCs). This term clearly distinguishes ieMMCs from lamina proprial MMCs (lpMMCs) while clearly communicating their common MC lineage. Both lpMMCs and ieMMCs are rare in normal mouse intestinal mucosa, but increased numbers of ieMMCs are seen as part of type 2 immune responses to intestinal helminth infections and in food allergies. Interestingly, we found that increased ieMMCs were consistently associated with decreased mucosal inflammation and damage, suggesting that they might have a role in controlling helminth-induced immunopathology. We also found that ieMMC hyperplasia can develop in the absence of helminth infections, for example, in Treg-deficient mice, Arf null mice, some nude mice, and certain graft-vs-host responses. Since tuft cell hyperplasia plays a critical role in type 2 immune responses to intestinal helminths, we looked for (but did not find) any direct relationship between ieMMC and tuft cell numbers in the intestinal mucosa. Much remains to be learned about the differing functions of ieMMCs and lpMMCs in the intestinal mucosa, but an essential step in deciphering their roles in mucosal immune responses will be to apply immunohistochemistry methods to consistently and accurately identify them in tissue sections.
Keywords: Heligmosomoides bakeri; Treg; Trichuris muris; graft-vs-host; immunohistochemistry; mast cell; mouse.
Figures






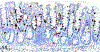

























Similar articles
-
Comparative Study of the Role of Interepithelial Mucosal Mast Cells in the Context of Intestinal Adenoma-Carcinoma Progression.Cancers (Basel). 2022 Apr 30;14(9):2248. doi: 10.3390/cancers14092248. Cancers (Basel). 2022. PMID: 35565377 Free PMC article.
-
Activation of mucosal mast cells promotes inflammation-related colon cancer development through recruiting and modulating inflammatory CD11b(+)Gr1(+) cells.Cancer Lett. 2015 Aug 10;364(2):173-80. doi: 10.1016/j.canlet.2015.05.014. Epub 2015 May 15. Cancer Lett. 2015. PMID: 25986744
-
Mast cell heterogeneity in various oral mucosal sites in the rat.Arch Oral Biol. 1992;37(6):445-50. doi: 10.1016/0003-9969(92)90098-s. Arch Oral Biol. 1992. PMID: 1637259
-
Mucosal Mast Cells as Key Effector Cells in Food Allergies.Cells. 2022 Jan 19;11(3):329. doi: 10.3390/cells11030329. Cells. 2022. PMID: 35159139 Free PMC article. Review.
-
New era for mucosal mast cells: their roles in inflammation, allergic immune responses and adjuvant development.Exp Mol Med. 2014 Mar 14;46(3):e83. doi: 10.1038/emm.2014.7. Exp Mol Med. 2014. PMID: 24626169 Free PMC article. Review.
Cited by
-
Tissue distribution of stem cell factor in adults.Exp Mol Pathol. 2021 Oct;122:104678. doi: 10.1016/j.yexmp.2021.104678. Epub 2021 Aug 24. Exp Mol Pathol. 2021. PMID: 34450114 Free PMC article.
-
Nonproliferative and Proliferative Lesions of the Rat and Mouse Hematolymphoid System.Toxicol Pathol. 2019 Aug;47(6):665-783. doi: 10.1177/0192623319867053. Toxicol Pathol. 2019. PMID: 31526133 Free PMC article.
-
Lymphoplasmacytic and eosinophilic enteritis with or without globule leukocyte hyperplasia in 4 goats.J Vet Intern Med. 2021 May;35(3):1620-1625. doi: 10.1111/jvim.16110. Epub 2021 May 2. J Vet Intern Med. 2021. PMID: 33934407 Free PMC article.
-
Correlations between clinical signs and corneal cytology in feline eosinophilic keratoconjunctivitis.Vet Ophthalmol. 2021 Nov;24(6):620-626. doi: 10.1111/vop.12909. Epub 2021 Jun 29. Vet Ophthalmol. 2021. PMID: 34184388 Free PMC article.
-
Comparative Study of the Role of Interepithelial Mucosal Mast Cells in the Context of Intestinal Adenoma-Carcinoma Progression.Cancers (Basel). 2022 Apr 30;14(9):2248. doi: 10.3390/cancers14092248. Cancers (Basel). 2022. PMID: 35565377 Free PMC article.
References
-
- Akpavie SO, Pirie HM. The globule leucocyte: morphology, origin, function and fate, a review. Anatomia, histologia, embryologia 1989;18(1):87–95. - PubMed
-
- Alizadeh H, Murrell KD. The intestinal mast cell response to Trichinella spiralis infection in mast cell-deficient w/wv mice. The Journal of parasitology. 1984;70(5):767–773. - PubMed
-
- Artis D, Villarino A, Silverman M, et al. The IL-27 receptor (WSX-1) is an inhibitor of innate and adaptive elements of type 2 immunity. Journal of immunology. 2004;173(9):5626–5634. - PubMed
-
- Austen KF. The heterogeneity of mast cell populations and products. Hospital practice. 1984;19(9):135–139, 143-136. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

