Production of fuels and chemicals from xylose by engineered Saccharomyces cerevisiae: a review and perspective
- PMID: 28494761
- PMCID: PMC5425999
- DOI: 10.1186/s12934-017-0694-9
Production of fuels and chemicals from xylose by engineered Saccharomyces cerevisiae: a review and perspective
Abstract
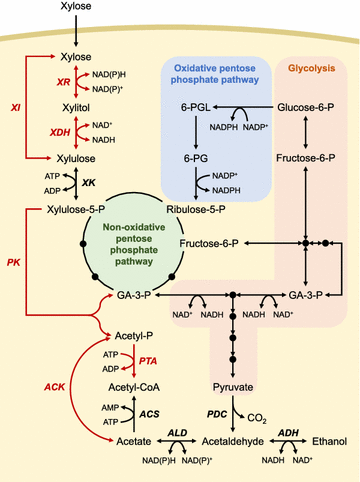
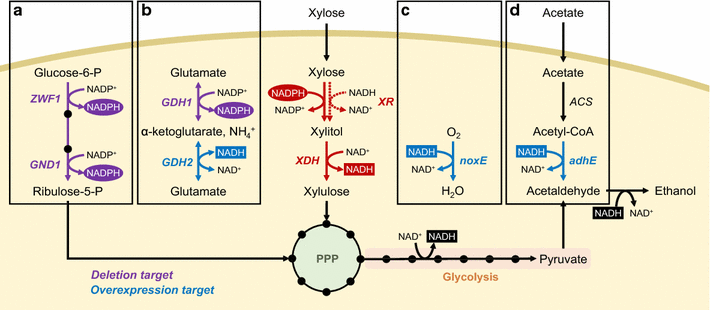
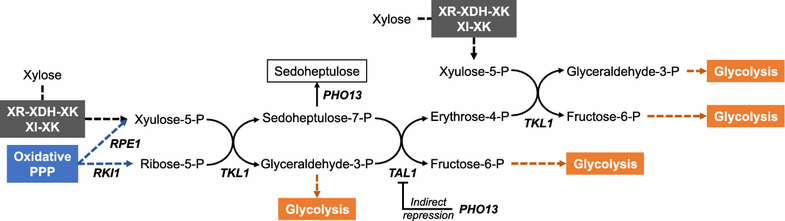
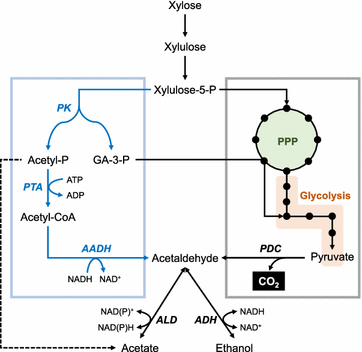
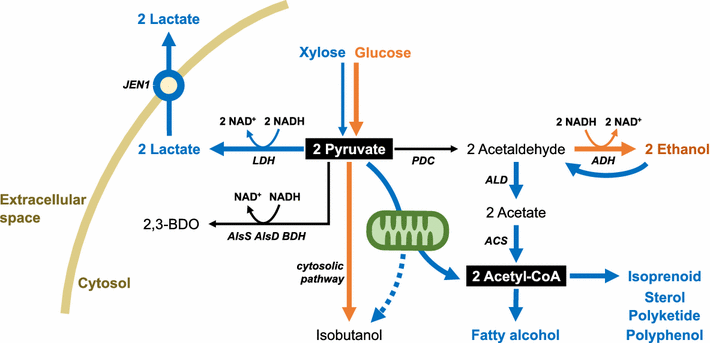
Efficient xylose utilization is one of the most important pre-requisites for developing an economic microbial conversion process of terrestrial lignocellulosic biomass into biofuels and biochemicals. A robust ethanol producing yeast Saccharomyces cerevisiae has been engineered with heterologous xylose assimilation pathways. A two-step oxidoreductase pathway consisting of NAD(P)H-linked xylose reductase and NAD+-linked xylitol dehydrogenase, and one-step isomerase pathway using xylose isomerase have been employed to enable xylose assimilation in engineered S. cerevisiae. However, the resulting engineered yeast exhibited inefficient and slow xylose fermentation. In order to improve the yield and productivity of xylose fermentation, expression levels of xylose assimilation pathway enzymes and their kinetic properties have been optimized, and additional optimizations of endogenous or heterologous metabolisms have been achieved. These efforts have led to the development of engineered yeast strains ready for the commercialization of cellulosic bioethanol. Interestingly, xylose metabolism by engineered yeast was preferably respiratory rather than fermentative as in glucose metabolism, suggesting that xylose can serve as a desirable carbon source capable of bypassing metabolic barriers exerted by glucose repression. Accordingly, engineered yeasts showed superior production of valuable metabolites derived from cytosolic acetyl-CoA and pyruvate, such as 1-hexadecanol and lactic acid, when the xylose assimilation pathway and target synthetic pathways were optimized in an adequate manner. While xylose has been regarded as a sugar to be utilized because it is present in cellulosic hydrolysates, potential benefits of using xylose instead of glucose for yeast-based biotechnological processes need to be realized.
Keywords: Metabolic engineering; Saccharomyces cerevisiae; Xylose.
Figures
Similar articles
-
Engineering of Saccharomyces cerevisiae for the efficient co-utilization of glucose and xylose.FEMS Yeast Res. 2017 Jun 1;17(4). doi: 10.1093/femsyr/fox034. FEMS Yeast Res. 2017. PMID: 28582494 Review.
-
Feasibility of xylose fermentation by engineered Saccharomyces cerevisiae overexpressing endogenous aldose reductase (GRE3), xylitol dehydrogenase (XYL2), and xylulokinase (XYL3) from Scheffersomyces stipitis.FEMS Yeast Res. 2013 May;13(3):312-21. doi: 10.1111/1567-1364.12036. Epub 2013 Mar 4. FEMS Yeast Res. 2013. PMID: 23398717
-
Enhanced xylose fermentation by engineered yeast expressing NADH oxidase through high cell density inoculums.J Ind Microbiol Biotechnol. 2017 Mar;44(3):387-395. doi: 10.1007/s10295-016-1899-3. Epub 2017 Jan 9. J Ind Microbiol Biotechnol. 2017. PMID: 28070721
-
Production of biofuels and chemicals from xylose using native and engineered yeast strains.Biotechnol Adv. 2019 Mar-Apr;37(2):271-283. doi: 10.1016/j.biotechadv.2018.12.003. Epub 2018 Dec 14. Biotechnol Adv. 2019. PMID: 30553928 Review.
-
Fermentation of mixed glucose-xylose substrates by engineered strains of Saccharomyces cerevisiae: role of the coenzyme specificity of xylose reductase, and effect of glucose on xylose utilization.Microb Cell Fact. 2010 Mar 10;9:16. doi: 10.1186/1475-2859-9-16. Microb Cell Fact. 2010. PMID: 20219100 Free PMC article.
Cited by
-
Metabolic engineering considerations for the heterologous expression of xylose-catabolic pathways in Saccharomyces cerevisiae.PLoS One. 2020 Jul 27;15(7):e0236294. doi: 10.1371/journal.pone.0236294. eCollection 2020. PLoS One. 2020. PMID: 32716960 Free PMC article.
-
Rational and evolutionary engineering of Saccharomyces cerevisiae for production of dicarboxylic acids from lignocellulosic biomass and exploring genetic mechanisms of the yeast tolerance to the biomass hydrolysate.Biotechnol Biofuels Bioprod. 2022 Feb 27;15(1):22. doi: 10.1186/s13068-022-02121-1. Biotechnol Biofuels Bioprod. 2022. PMID: 35219341 Free PMC article.
-
Production of ethanol fuel from enzyme-treated sugarcane bagasse hydrolysate using d-xylose-fermenting wild yeast isolated from Brazilian biomes.3 Biotech. 2018 Jul;8(7):312. doi: 10.1007/s13205-018-1340-x. Epub 2018 Jul 11. 3 Biotech. 2018. PMID: 30023144 Free PMC article.
-
Association of improved oxidative stress tolerance and alleviation of glucose repression with superior xylose-utilization capability by a natural isolate of Saccharomyces cerevisiae.Biotechnol Biofuels. 2018 Feb 5;11:28. doi: 10.1186/s13068-018-1018-y. eCollection 2018. Biotechnol Biofuels. 2018. PMID: 29441126 Free PMC article.
-
Screening and evolution of a novel protist xylose isomerase from the termite Reticulitermes speratus for efficient xylose fermentation in Saccharomyces cerevisiae.Biotechnol Biofuels. 2017 Aug 23;10:203. doi: 10.1186/s13068-017-0890-1. eCollection 2017. Biotechnol Biofuels. 2017. PMID: 28852424 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

