Reducing depressive symptomatology with a smartphone app: study protocol for a randomized, placebo-controlled trial
- PMID: 28494802
- PMCID: PMC5427551
- DOI: 10.1186/s13063-017-1960-1
Reducing depressive symptomatology with a smartphone app: study protocol for a randomized, placebo-controlled trial
Abstract
Background: Depression has become one of the leading contributors to the global disease burden. Evidence-based treatments for depression are available, but access to them is still limited in some instances. As technology has become more integrated into mental health care, computerized cognitive behavioral therapy (CBT) protocols have become available and have been recently transposed to mobile environments (e.g., smartphones) in the form of "apps." Preliminary research on some depression apps has shown promising results in reducing subthreshold or mild to moderate depressive symptoms. However, this small number of studies reports a low statistical power and they have not yet been replicated. Moreover, none of them included an active placebo comparison group. This is problematic, as a "digital placebo effect" may explain some of the positive effects documented until now. The aim of this study is to test a newly developed mobile app firmly grounded in the CBT theory of depression to determine whether this app is clinically useful in decreasing moderate depressive symptoms when compared with an active placebo. Additionally, we are interested in the app's effect on emotional wellbeing and depressogenic cognitions.
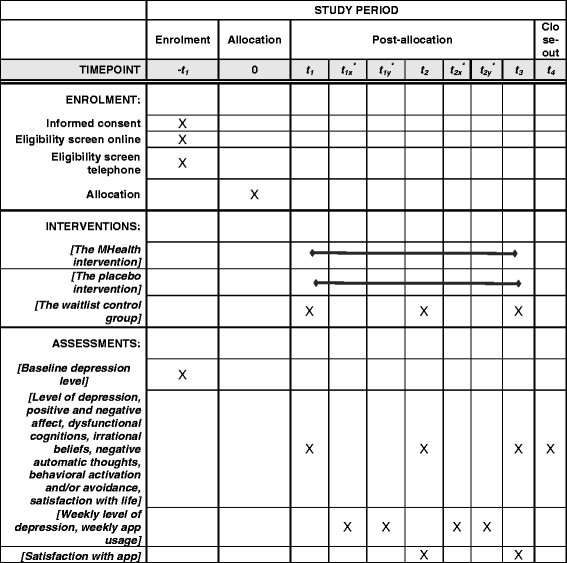
Methods/design: Romanian-speaking adults (18 years and older) with access to a computer and the Internet and owning a smartphone are included in the study. A randomized, three-arm clinical trial is being conducted (i.e., active intervention, placebo intervention and delayed intervention). Two hundred and twenty participants with moderate depressive symptoms (i.e., obtaining scores >9 and ≤16 on the Patient Health Questionnaire, PHQ-9) will be randomized to the three conditions. Participants undergoing therapy, presenting serious mental health problems, or legal or health issues that would prevent them from using the app, as well as participants reporting suicidal ideation are excluded. Participants randomized to the active and placebo interventions will use the smartphone app for 6 weeks. A short therapist check-in via phone will take place every week. Participants in the delayed-intervention condition will be given access to the app after 6 weeks from randomization. The primary outcome is the level of depressive symptomatology. The intervention delivered through the app to the active condition includes psychoeducational materials and exercises based on CBT for depression, while the placebo intervention uses a sham version of the app (i.e., similar structure of courses and exercises).
Discussion: To our knowledge, this study protocol is the first to test the efficacy of a smartphone app for depressive symptomatology in the form of a randomized controlled trial (RCT) that includes an active placebo condition. As such, this can substantially add to the body of evidence supporting the use of apps designed to decrease depression.
Trial registration: ClinicalTrials.gov, identifier: NCT03060200 . Registered on 1 February 2017. The first participant was enrolled on 17 February 2017.
Keywords: CBT; Depression; Placebo; Protocol; Randomized trial; Smartphone app.
Figures

References
-
- World Health Organization. Policies and practices for mental health in Europe. Meeting the challenges. 2008. http://www.euro.who.int/en/publications/abstracts/policies-and-practices.... Accessed 17 Aug 2015.
-
- WHO. Depression facts sheet. http://www.who.int/en/. 2012. http://www.who.int/mediacentre/factsheets/fs369/en/. Accessed 11 May 2013.
-
- Deslich S, Stec B, Tomblin S, Coustasse A. Telepsychiatry in the 21st century: transforming healthcare with technology. Perspect Health Inf Manag AHIMA Am Health Inf Manag Assoc. 2013;10. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3709879/. Accessed 27 Sep 2016. - PMC - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

