Rate-Dependent Role of IKur in Human Atrial Repolarization and Atrial Fibrillation Maintenance
- PMID: 28494969
- PMCID: PMC5425383
- DOI: 10.1016/j.bpj.2017.03.022
Rate-Dependent Role of IKur in Human Atrial Repolarization and Atrial Fibrillation Maintenance
Abstract
The atrial-specific ultrarapid delayed rectifier K+ current (IKur) inactivates slowly but completely at depolarized voltages. The consequences for IKur rate-dependence have not been analyzed in detail and currently available mathematical action-potential (AP) models do not take into account experimentally observed IKur inactivation dynamics. Here, we developed an updated formulation of IKur inactivation that accurately reproduces time-, voltage-, and frequency-dependent inactivation. We then modified the human atrial cardiomyocyte Courtemanche AP model to incorporate realistic IKur inactivation properties. Despite markedly different inactivation dynamics, there was no difference in AP parameters across a wide range of stimulation frequencies between the original and updated models. Using the updated model, we showed that, under physiological stimulation conditions, IKur does not inactivate significantly even at high atrial rates because the transmembrane potential spends little time at voltages associated with inactivation. Thus, channel dynamics are determined principally by activation kinetics. IKur magnitude decreases at higher rates because of AP changes that reduce IKur activation. Nevertheless, the relative contribution of IKur to AP repolarization increases at higher frequencies because of reduced activation of the rapid delayed-rectifier current IKr. Consequently, IKur block produces dose-dependent termination of simulated atrial fibrillation (AF) in the absence of AF-induced electrical remodeling. The inclusion of AF-related ionic remodeling stabilizes simulated AF and greatly reduces the predicted antiarrhythmic efficacy of IKur block. Our results explain a range of experimental observations, including recently reported positive rate-dependent IKur-blocking effects on human atrial APs, and provide insights relevant to the potential value of IKur as an antiarrhythmic target for the treatment of AF.
Copyright © 2017 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures



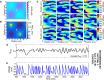
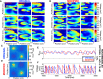


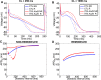
References
-
- Wang Z., Fermini B., Nattel S. Sustained depolarization-induced outward current in human atrial myocytes. Evidence for a novel delayed rectifier K+ current similar to Kv1.5 cloned channel currents. Circ. Res. 1993;73:1061–1076. - PubMed
-
- Feng J., Xu D., Nattel S. Ultrarapid delayed rectifier current inactivation in human atrial myocytes: properties and consequences. Am. J. Physiol. 1998;275:H1717–H1725. - PubMed
-
- Courtemanche M., Ramirez R.J., Nattel S. Ionic mechanisms underlying human atrial action potential properties: insights from a mathematical model. Am. J. Physiol. 1998;275:H301–H321. - PubMed
-
- Kneller J., Zou R., Nattel S. Cholinergic atrial fibrillation in a computer model of a two-dimensional sheet of canine atrial cells with realistic ionic properties. Circ. Res. 2002;90:E73–E87. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

