Quantification of saxitoxin in human blood by ELISA
- PMID: 28495477
- PMCID: PMC6234504
- DOI: 10.1016/j.toxicon.2017.05.009
Quantification of saxitoxin in human blood by ELISA
Abstract
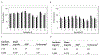
Saxitoxin (STX) is a potent marine toxin that causes paralytic shellfish poisoning (PSP) which can result in significant morbidity and mortality in humans. Low lethal doses, rapid onset of PSP symptoms, and brief STX half-life in vivo require sensitive and rapid diagnostic techniques to monitor human exposures. Our laboratory has validated an enzyme-linked immunosorbent assay (ELISA) for quantitative detection of STX from 0.020 to 0.80 ng/mL in human whole blood and from 0.06 to 2.0 ng/mL in dried human blood which is simple, sensitive, rapid, and cost-effective. To our knowledge, this is the first validated method for the quantitation of saxitoxin in whole blood. Microsampling devices were used in sample collection which allows for standardized collection of blood, stable storage, and cost-efficient shipping. Quality control precision and accuracy were evaluated over the course of validation and were within 20% of theoretical concentrations. No detectable background concentrations of STX were found among fifty whole blood and dried blood convenience samples. Additionally, ten spiked individual whole blood and dried blood samples were tested for accuracy and precision and were within 20% of theoretical concentrations. Gonyautoxins 2&3 (GTX2&3) cross-reacted with this ELISA by 21%, but all other structurally related PSP toxins tested cross-reacted less than two percent. While clinical diagnosis or treatment of PSP would be unaffected by GTX2&3 cross-reactivity by ELISA, to accurately quantify individual PSP toxins, these results should be coupled with high performance liquid chromatography mass spectrometry measurements.
Keywords: Dried blood; ELISA; Microsampling devices; Saxitoxin.
Published by Elsevier Ltd.
Figures
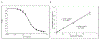
References
-
- Ainsbury EA, Bakhanova E, Barquinero JF, Brai M, Chumak V, Correcher V, Darroudi F, Fattibene P, Gruel G, Guclu I, Horn S, Jaworska A, Kulka U, Lindholm C, Lloyd D, Longo A, Marrale M, Monteiro Gil O, Oestreicher U, Pajic J, Rakic B, Romm H, Trompier F, Veronese I, Voisin P, Vral A, Whitehouse CA, Wieser A, Woda C, Wojcik A, Rothkamm K, 2011. Review of retrospective dosimetry techniques for external ionising radiation exposures. Radiat. Prot. Dosim 147 (4), 573–592. - PubMed
-
- Bragg WA, Lemire SW, Coleman RM, Hamelin EI, Johnson RC, 2015. Detection of human exposure to saxitoxin and neosaxitoxin in urine by online-solid phase extraction-liquid chromatography-tandem mass spectrometry. Toxicon Off. J. Int. Soc. Toxinol 99, 118–124. - PubMed
-
- DeGrasse Stacey, Rivera Victor, Roach John, White Kevin, Callahan John, Couture Darcie, Simone Karen, Peredy Tamas, Poli Mark, 2014. Paralytic shellfish toxins in clinical matrices: extension of AOAC official method 2005.06 to human urine and serum and application to a 2007 case study in Maine. Deep Sea Res. Part II Top. Stud. Oceanogr 103, 368–375.
-
- Doucette Gregory J., Logan Margaret M., Ramsdell John S., Van Dolah Frances M., 1997. Development and preliminary validation of a microtiter plate-based receptor binding assay for paralytic shellfish poisoning toxins. Toxicon 35 (5), 625–636. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

