Concurrent activation of β2-adrenergic receptor and blockage of GPR55 disrupts pro-oncogenic signaling in glioma cells
- PMID: 28495590
- PMCID: PMC5512002
- DOI: 10.1016/j.cellsig.2017.05.006
Concurrent activation of β2-adrenergic receptor and blockage of GPR55 disrupts pro-oncogenic signaling in glioma cells
Abstract
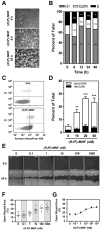
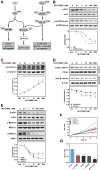
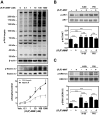
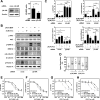
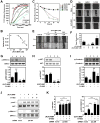
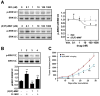
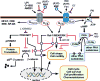
Activation of β2-adrenergic receptor (β2AR) and deorphanized GPR55 has been shown to modulate cancer growth in diverse tumor types in vitro and in xenograft models in vivo. (R,R')-4'-methoxy-1-naphthylfenoterol [(R,R')-MNF] is a bivalent compound that agonizes β2AR but inhibits GPR55-mediated pro-oncogenic responses. Here, we investigated the molecular mechanisms underlying the anti-tumorigenic effects of concurrent β2AR activation and GPR55 blockade in C6 glioma cells using (R,R')-MNF as a marker ligand. Our data show that (R,R')-MNF elicited G1-phase cell cycle arrest and apoptosis, reduced serum-inducible cell motility, promoted the phosphorylation of PKA target proteins, and inhibited constitutive activation of ERK and AKT in the low nanomolar range, whereas high nanomolar levels of (R,R')-MNF were required to block GPR55-mediated cell motility. siRNA knockdown and pharmacological inhibition of β2AR activity were accompanied by significant upregulation of AKT and ERK phosphorylation, and selective alteration in (R,R')-MNF responsiveness. The effects of agonist stimulation of GPR55 on various readouts, including cell motility assays, were suppressed by (R,R')-MNF. Lastly, a significant increase in phosphorylation-mediated inactivation of β-catenin occurred with (R,R')-MNF, and we provided new evidence of (R,R')-MNF-mediated inhibition of oncogenic β-catenin signaling in a C6 xenograft tumor model. Thus, simultaneous activation of β2AR and blockade of GPR55 may represent a novel therapeutic approach to combat the progression of glioblastoma cancer.
Keywords: Bivalent ligand; Brain cancer; Cannabinoid receptor; Fenoterol derivative; Invasiveness.
Published by Elsevier Inc.
Conflict of interest statement
Drs. Bernier and Wainer are listed as co-inventors on a patent for the use of fenoterol and fenoterol analogues, including (
Figures

Similar articles
-
GPR55 receptor antagonist decreases glycolytic activity in PANC-1 pancreatic cancer cell line and tumor xenografts.Int J Cancer. 2017 Nov 15;141(10):2131-2142. doi: 10.1002/ijc.30904. Epub 2017 Aug 7. Int J Cancer. 2017. PMID: 28741686 Free PMC article.
-
Activation of β2-adrenergic receptor by (R,R')-4'-methoxy-1-naphthylfenoterol inhibits proliferation and motility of melanoma cells.Cell Signal. 2015 May;27(5):997-1007. doi: 10.1016/j.cellsig.2015.02.012. Epub 2015 Feb 20. Cell Signal. 2015. PMID: 25703025 Free PMC article.
-
Selective GPR55 antagonism reduces chemoresistance in cancer cells.Pharmacol Res. 2016 Sep;111:757-766. doi: 10.1016/j.phrs.2016.07.013. Epub 2016 Jul 14. Pharmacol Res. 2016. PMID: 27423937 Free PMC article.
-
The oncogenic lysophosphatidylinositol (LPI)/GPR55 signaling.Life Sci. 2022 Jul 15;301:120596. doi: 10.1016/j.lfs.2022.120596. Epub 2022 Apr 30. Life Sci. 2022. PMID: 35500681 Review.
-
Effect of fenoterol stereochemistry on the β2 adrenergic receptor system: ligand-directed chiral recognition.Chirality. 2011;23 Suppl 1(Suppl 1):E1-6. doi: 10.1002/chir.20963. Epub 2011 May 26. Chirality. 2011. PMID: 21618615 Free PMC article. Review.
Cited by
-
Nuclear size rectification: A potential new therapeutic approach to reduce metastasis in cancer.Front Cell Dev Biol. 2022 Oct 10;10:1022723. doi: 10.3389/fcell.2022.1022723. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36299481 Free PMC article. Review.
-
β adrenergic receptor modulated signaling in glioma models: promoting β adrenergic receptor-β arrestin scaffold-mediated activation of extracellular-regulated kinase 1/2 may prove to be a panacea in the treatment of intracranial and spinal malignancy and extra-neuraxial carcinoma.Mol Biol Rep. 2020 Jun;47(6):4631-4650. doi: 10.1007/s11033-020-05427-1. Epub 2020 Apr 18. Mol Biol Rep. 2020. Retraction in: Mol Biol Rep. 2022 Nov;49(11):11215. doi: 10.1007/s11033-022-07301-8. PMID: 32303958 Free PMC article. Retracted. Review.
-
Structural Insights into Ligand-Receptor Interactions Involved in Biased Agonism of G-Protein Coupled Receptors.Molecules. 2021 Feb 6;26(4):851. doi: 10.3390/molecules26040851. Molecules. 2021. PMID: 33561962 Free PMC article. Review.
-
GPR55 receptor antagonist decreases glycolytic activity in PANC-1 pancreatic cancer cell line and tumor xenografts.Int J Cancer. 2017 Nov 15;141(10):2131-2142. doi: 10.1002/ijc.30904. Epub 2017 Aug 7. Int J Cancer. 2017. PMID: 28741686 Free PMC article.
-
Evaluating the Antitumor Potential of Cannabichromene, Cannabigerol, and Related Compounds from Cannabis sativa and Piper nigrum Against Malignant Glioma: An In Silico to In Vitro Approach.Int J Mol Sci. 2025 Jun 13;26(12):5688. doi: 10.3390/ijms26125688. Int J Mol Sci. 2025. PMID: 40565152 Free PMC article.
References
-
- Korolev KS, Xavier JB, Gore J. Nat Rev Cancer. 2014;14:371–380. - PubMed
-
- Shipitsin M, Campbell LL, Argani P, Weremowicz S, Bloushtain-Qimron N, Yao J, Nikolskaya T, Serebryiskaya T, Beroukhim R, Hu M, Halushka MK, Sukumar S, Parker LM, Anderson KS, Harris LN, Garber JE, Richardson AL, Schnitt SJ, Nikolsky Y, Gelman RS, Polyak K. Cancer Cell. 2007;11:259–273. - PubMed
-
- Vogel CL, Cobleigh MA, Tripathy D, Gutheil JC, Harris LN, Fehrenbacher L, Slamon DJ, Murphy M, Novotny WF, Burchmore M, Shak S, Stewart SJ, Press M. J Clin Oncol. 2002;20:719–726. - PubMed
-
- Druker BJ. Nat Med. 2009;15:1149–1152. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

