Mechanically patterned neuromuscular junctions-in-a-dish have improved functional maturation
- PMID: 28495800
- PMCID: PMC5541845
- DOI: 10.1091/mbc.E17-01-0046
Mechanically patterned neuromuscular junctions-in-a-dish have improved functional maturation
Abstract
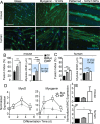
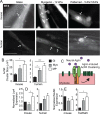
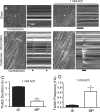
Motor neuron (MN) diseases are progressive disorders resulting from degeneration of neuromuscular junctions (NMJs), which form the connection between MNs and muscle fibers. NMJ-in-a-dish models have been developed to examine human MN-associated dysfunction with disease; however such coculture models have randomly oriented myotubes with immature synapses that contract asynchronously. Mechanically patterned (MP) extracellular matrix with alternating soft and stiff stripes improves current NMJ-in-a-dish models by inducing both mouse and human myoblast durotaxis to stripes where they aligned, differentiated, and fused into patterned myotubes. Compared to conventional culture on rigid substrates or unpatterned hydrogels, MP substrates supported increased differentiation and fusion, significantly larger acetylcholine (ACh) receptor clusters, and increased expression of MuSK and Lrp4, two cell surface receptors required for NMJ formation. Robust contractions were observed when mouse myotubes were stimulated by ACh, with twitch duration and frequency most closely resembling those for mature muscle on MP substrates. Fused myotubes, when cocultured with MNs, were able to form even larger NMJs. Thus MP matrices produce more functionally active NMJs-in-a-dish, which could be used to elucidate disease pathology and facilitate drug discovery.
© 2017 Happe et al. This article is distributed by The American Society for Cell Biology under license from the author(s). Two months after publication it is available to the public under an Attribution–Noncommercial–Share Alike 3.0 Unported Creative Commons License (http://creativecommons.org/licenses/by-nc-sa/3.0).
Figures




References
-
- Altomare L, Riehle M, Gadegaard N, Tanzi MC, Fare S. Microcontact printing of fibronectin on a biodegradable polymeric surface for skeletal muscle cell orientation. Int J Artif Organs. 2010;33:535–543. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

