Randomised controlled trial of online continuing education for health professionals to improve the management of chronic fatigue syndrome: a study protocol
- PMID: 28495811
- PMCID: PMC5541332
- DOI: 10.1136/bmjopen-2016-014133
Randomised controlled trial of online continuing education for health professionals to improve the management of chronic fatigue syndrome: a study protocol
Abstract
Introduction: Chronic fatigue syndrome (CFS) is a serious and debilitating illness that affects between 0.2%-2.6% of the world's population. Although there is level 1 evidence of the benefit of cognitive behaviour therapy (CBT) and graded exercise therapy (GET) for some people with CFS, uptake of these interventions is low or at best untimely. This can be partly attributed to poor clinician awareness and knowledge of CFS and related CBT and GET interventions. This trial aims to evaluate the effect of participation in an online education programme, compared with a wait-list control group, on allied health professionals' knowledge about evidence-based CFS interventions and their levels of confidence to engage in the dissemination of these interventions.
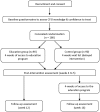
Methods and analysis: A randomised controlled trial consisting of 180 consenting allied health professionals will be conducted. Participants will be randomised into an intervention group (n=90) that will receive access to the online education programme, or a wait-list control group (n=90). The primary outcomes will be: 1) knowledge and clinical reasoning skills regarding CFS and its management, measured at baseline, postintervention and follow-up, and 2) self-reported confidence in knowledge and clinical reasoning skills related to CFS. Secondary outcomes include retention of knowledge and satisfaction with the online education programme. The influence of the education programme on clinical practice behaviour, and self-reported success in the management of people with CFS, will also be assessed in a cohort study design with participants from the intervention and control groups combined.
Ethics and dissemination: The study protocol has been approved by the Human Research Ethics Committee at The University of New South Wales (approval number HC16419). Results will be disseminated via peer-reviewed journal articles and presentations at scientific conferences and meetings.
Trial registration: ACTRN12616000296437.
Keywords: chronic fatigue syndrome; cognitive behaviour therapy; education; graded exercise therapy; online; randomised controlled trial.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2017. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Conflict of interest statement
Competing interests: None declared.
Similar articles
-
Does a fall prevention educational programme improve knowledge and change exercise prescribing behaviour in health and exercise professionals? A study protocol for a randomised controlled trial.BMJ Open. 2014 Nov 19;4(11):e007032. doi: 10.1136/bmjopen-2014-007032. BMJ Open. 2014. PMID: 25410607 Free PMC article. Clinical Trial.
-
Investigating the effectiveness and cost-effectiveness of FITNET-NHS (Fatigue In Teenagers on the interNET in the NHS) compared to Activity Management to treat paediatric chronic fatigue syndrome (CFS)/myalgic encephalomyelitis (ME): protocol for a randomised controlled trial.Trials. 2018 Feb 22;19(1):136. doi: 10.1186/s13063-018-2500-3. Trials. 2018. PMID: 29471861 Free PMC article.
-
Study protocol of the TIRED study: a randomised controlled trial comparing either graded exercise therapy for severe fatigue or cognitive behaviour therapy with usual care in patients with incurable cancer.BMC Cancer. 2017 Jan 28;17(1):81. doi: 10.1186/s12885-017-3076-0. BMC Cancer. 2017. PMID: 28129746 Free PMC article. Clinical Trial.
-
A review on cognitive behavorial therapy (CBT) and graded exercise therapy (GET) in myalgic encephalomyelitis (ME) / chronic fatigue syndrome (CFS): CBT/GET is not only ineffective and not evidence-based, but also potentially harmful for many patients with ME/CFS.Neuro Endocrinol Lett. 2009;30(3):284-99. Neuro Endocrinol Lett. 2009. PMID: 19855350 Review.
-
Cost-effectiveness of Interventions for Chronic Fatigue Syndrome or Myalgic Encephalomyelitis: A Systematic Review of Economic Evaluations.Appl Health Econ Health Policy. 2021 Jul;19(4):473-486. doi: 10.1007/s40258-021-00635-7. Epub 2021 Mar 1. Appl Health Econ Health Policy. 2021. PMID: 33646528 Free PMC article.
Cited by
-
Association between medical students' prior experiences and perceptions of formal online education developed in response to COVID-19: a cross-sectional study in China.BMJ Open. 2020 Oct 29;10(10):e041886. doi: 10.1136/bmjopen-2020-041886. BMJ Open. 2020. PMID: 33122327 Free PMC article.
-
A Mobile Phone Text Messaging Intervention to Manage Fatigue for People With Multiple Sclerosis, Spinal Cord Injury, and Stroke: Development and Usability Testing.JMIR Form Res. 2022 Dec 21;6(12):e40166. doi: 10.2196/40166. JMIR Form Res. 2022. PMID: 36542466 Free PMC article.
References
-
- Fukuda K, Straus SE, Hickie I, et al. . The chronic fatigue syndrome: a comprehensive approach to its definition and study. International chronic Fatigue syndrome Study Group. Ann Intern Med 1994;121:953–9. - PubMed
-
- Castell BD, Kazantzis N, Moss-Morris RE. Cognitive behavioral therapy and graded exercise for chronic Fatigue syndrome: a Meta-Analysis. Clinical Psychology: Science and Practice 2011;18:311–24.10.1111/j.1468-2850.2011.01262.x - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical