Determinants of treatment duration in the prevention of recurrent venous thromboembolism: a protocol for a balanced vignette experiment
- PMID: 28495816
- PMCID: PMC5777455
- DOI: 10.1136/bmjopen-2016-015231
Determinants of treatment duration in the prevention of recurrent venous thromboembolism: a protocol for a balanced vignette experiment
Abstract
Introduction: Venous thromboembolism (VTE) is a condition that annually occurs in approximately 1‰ of the world's population. Patients who have already had a VTE are at elevated risk for a recurrent VTE. Recurrent events increase the risk of long-term sequelae and can be fatal. Adequate secondary prophylaxis is thus needed to prevent such events. Patients with VTE are often prone to bleeding, and pharmacological prophylaxis exacerbates bleeding risk. Expert opinions on the optimum duration of secondary prophylaxis in VTE still vary substantially. The existence of treatment guidelines has not led to uniformity of VTE secondary prophylaxis strategies, which means that physicians still adhere to individual risk calculi in determining treatment duration.
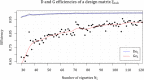
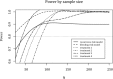
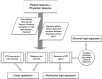
Methods and analysis: The aim of this study is to establish what factors lie at the root of this variance in VTE secondary prophylactic treatment strategies, and what risk factors are deemed of particular importance in determining the perceived risks and benefits of variable treatment durations. To do this, we created a survey based on a D-efficient and G-efficient balanced experimental vignette design. This protocol covers all aspects of how this survey was set up and how it was implemented. The analysis of the experimental data will be carried out using mixed-effects methods, which are beneficial in scenarios with high interindividual variance and correlated (eg, repeated-measures) responses. We propose the use of maximal random effects structures insofar as possible.
Ethics and dissemination: All data are de-identified, and any identifying characteristics of the respondents will not be reported in a final manuscript or elsewhere. A paper describing the expert interviews is currently under peer review. A manuscript that contains the analysis of the results of the experiment described in this protocol is being drafted, and will also be submitted to a peer-reviewed journal.
Keywords: Duration of Treatment; Secondary Prophylaxis; Surveys and Questionnaires; Venous Thromboembolism.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2017. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Conflict of interest statement
Competing interests: VTC and BABE declare no competing interests. MHP has received research support and honoraria, and participated in advisory boards for Bayer HealthCare, Sanofi-Aventis, Boehringer Ingelheim, GlaxoSmithKline, Daiichi Sankyo, LeoPharma, ThromboGenics, and Pfizer.
Figures
Similar articles
-
Venous thromboembolism, thrombophilia, antithrombotic therapy, and pregnancy: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition).Chest. 2008 Jun;133(6 Suppl):844S-886S. doi: 10.1378/chest.08-0761. Chest. 2008. PMID: 18574280
-
Extended duration of anticoagulation with edoxaban in patients with venous thromboembolism: a post-hoc analysis of the Hokusai-VTE study.Lancet Haematol. 2016 May;3(5):e228-36. doi: 10.1016/S2352-3026(16)00023-5. Epub 2016 Mar 22. Lancet Haematol. 2016. PMID: 27132697 Clinical Trial.
-
Extended therapy for primary and secondary prevention of venous thromboembolism.J Pharm Pract. 2010 Aug;23(4):313-23. doi: 10.1177/0897190010366930. Epub 2010 May 7. J Pharm Pract. 2010. PMID: 21507831 Review.
-
International clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer.J Thromb Haemost. 2013 Jan;11(1):56-70. doi: 10.1111/jth.12070. J Thromb Haemost. 2013. PMID: 23217107 Review.
-
Chemical venous thromboembolic prophylaxis is safe and effective for patients with traumatic brain injury when started 24 hours after the absence of hemorrhage progression on head CT.J Trauma Acute Care Surg. 2012 Aug;73(2):426-30. doi: 10.1097/TA.0b013e31825a758b. J Trauma Acute Care Surg. 2012. PMID: 22846950
Cited by
-
Extended anticoagulant therapy in venous thromboembolism: a balanced, fractional factorial, clinical vignette-based study.Haematologica. 2019 Oct;104(10):e474-e477. doi: 10.3324/haematol.2018.209924. Epub 2019 Mar 7. Haematologica. 2019. PMID: 30846497 Free PMC article. No abstract available.
-
A Nomogram for Predicting Risk of Thromboembolism in Gastric Cancer Patients Receiving Chemotherapy.Front Oncol. 2021 May 26;11:598116. doi: 10.3389/fonc.2021.598116. eCollection 2021. Front Oncol. 2021. PMID: 34123774 Free PMC article.
References
-
- Baser O. Prevalence and economic burden of venous thromboembolism after total hip arthroplasty or total knee arthroplasty. Am J Manag Care 2011;17:S6–S8. - PubMed
-
- Zhu T, Martinez I, Emmerich J, et al. Treatment, and public awareness. Arterioscl Throm Vas 2009;29:298–310. - PubMed
-
- Beyer-Westendorf J, Cohen AT, Monreal M. Venous thromboembolism prevention and treatment: expanding the rivaroxaban knowledge base with real-life data. Eur Heart J Supp 2015;17:D32–D41. 10.1093/eurheartj/suv034 - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical