Hyperbaric Oxygen Reduces Infarction Volume and Hemorrhagic Transformation Through ATP/NAD+/Sirt1 Pathway in Hyperglycemic Middle Cerebral Artery Occlusion Rats
- PMID: 28495827
- PMCID: PMC5489129
- DOI: 10.1161/STROKEAHA.116.015753
Hyperbaric Oxygen Reduces Infarction Volume and Hemorrhagic Transformation Through ATP/NAD+/Sirt1 Pathway in Hyperglycemic Middle Cerebral Artery Occlusion Rats
Abstract
Background and purpose: Energy depletion is a critical factor leading to cell death and brain dysfunction after ischemic stroke. In this study, we investigated whether energy depletion is involved in hyperglycemia-induced hemorrhagic transformation after ischemic stroke and determined the pathway underlying the beneficial effects of hyperbaric oxygen (HBO).
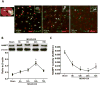
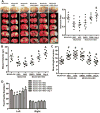
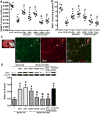
Methods: After 2-hour middle cerebral artery occlusion, hyperglycemia was induced by injecting 50% dextrose (6 mL/kg) intraperitoneally at the onset of reperfusion. Immediately after it, rats were exposed to HBO at 2 atmospheres absolutes for 1 hour. ATP synthase inhibitor oligomycin A, nicotinamide phosphoribosyl transferase inhibitor FK866, or silent mating type information regulation 2 homolog 1 siRNA was administrated for interventions. Infarct volume, hemorrhagic volume, and neurobehavioral deficits were recorded; the level of blood glucose, ATP, and nicotinamide adenine dinucleotide and the activity of nicotinamide phosphoribosyl transferase were monitored; the expression of silent mating type information regulation 2 homolog 1, acetylated p53, acetylated nuclear factor-κB, and cleaved caspase 3 were detected by Western blots; and the activity of matrix metalloproteinase-9 was assayed by zymography.
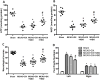
Results: Hyperglycemia deteriorated energy metabolism and reduced the level of ATP and nicotinamide adenine dinucleotide and exaggerated hemorrhagic transformation, blood-brain barrier disruption, and neurological deficits after middle cerebral artery occlusion. HBO treatment increased the levels of the ATP and nicotinamide adenine dinucleotide and consequently increased silent mating type information regulation 2 homolog 1, resulting in attenuation of hemorrhagic transformation, brain infarction, as well as improvement of neurological function in hyperglycemic middle cerebral artery occlusion rats.
Conclusions: HBO induced activation of ATP/nicotinamide adenine dinucleotide/silent mating type information regulation 2 homolog 1 pathway and protected blood-brain barrier in hyperglycemic middle cerebral artery occlusion rats. HBO might be promising approach for treatment of acute ischemic stroke patients, especially patients with diabetes mellitus or treated with r-tPA (recombinant tissue-type plasminogen activator).
Keywords: NAD; NAMPT; Sirt1 and hemorrhagic transformation; hyperbaric oxygenation; stroke.
© 2017 American Heart Association, Inc.
Figures

Similar articles
-
Hyperbaric oxygen-induced attenuation of hemorrhagic transformation after experimental focal transient cerebral ischemia.Stroke. 2007 Apr;38(4):1362-7. doi: 10.1161/01.STR.0000259660.62865.eb. Epub 2007 Feb 22. Stroke. 2007. PMID: 17322079
-
Hyperbaric Oxygen Preconditioning Attenuates Hemorrhagic Transformation Through Reactive Oxygen Species/Thioredoxin-Interacting Protein/Nod-Like Receptor Protein 3 Pathway in Hyperglycemic Middle Cerebral Artery Occlusion Rats.Crit Care Med. 2016 Jun;44(6):e403-11. doi: 10.1097/CCM.0000000000001468. Crit Care Med. 2016. PMID: 26646457
-
Hyperbaric oxygen preconditioning attenuates hyperglycemia-enhanced hemorrhagic transformation by inhibiting matrix metalloproteinases in focal cerebral ischemia in rats.Exp Neurol. 2013 Sep;247:737-43. doi: 10.1016/j.expneurol.2013.03.019. Epub 2013 Mar 26. Exp Neurol. 2013. PMID: 23537951 Free PMC article.
-
The Efficacy of Hyperbaric Oxygen Therapy on Middle Cerebral Artery Occlusion in Animal Studies: A Meta-Analysis.PLoS One. 2016 Feb 9;11(2):e0148324. doi: 10.1371/journal.pone.0148324. eCollection 2016. PLoS One. 2016. PMID: 26859390 Free PMC article.
-
Impaired nicotinamide adenine dinucleotide (NAD+ ) metabolism in diabetes and diabetic tissues: Implications for nicotinamide-related compound treatment.J Diabetes Investig. 2020 Nov;11(6):1403-1419. doi: 10.1111/jdi.13303. Epub 2020 Jul 7. J Diabetes Investig. 2020. PMID: 32428995 Free PMC article. Review.
Cited by
-
Melatonin protects against focal cerebral ischemia-reperfusion injury in diabetic mice by ameliorating mitochondrial impairments: involvement of the Akt-SIRT3-SOD2 signaling pathway.Aging (Albany NY). 2021 Jun 11;13(12):16105-16123. doi: 10.18632/aging.203137. Epub 2021 Jun 11. Aging (Albany NY). 2021. PMID: 34118791 Free PMC article.
-
Effects and mechanisms of CTRP3 overexpression in secondary brain injury following intracerebral hemorrhage in rats.Exp Ther Med. 2022 Jan;23(1):35. doi: 10.3892/etm.2021.10957. Epub 2021 Nov 9. Exp Ther Med. 2022. PMID: 34849150 Free PMC article.
-
A Dual Role for Hyperbaric Oxygen in Stroke Neuroprotection: Preconditioning of the Brain and Stem Cells.Cond Med. 2018 Jun;1(4):151-166. Cond Med. 2018. PMID: 30079404 Free PMC article.
-
Trifluoperazine regulates blood-brain barrier permeability via the MLCK/p-MLC pathway to promote ischemic stroke recovery.iScience. 2024 Feb 13;27(3):109156. doi: 10.1016/j.isci.2024.109156. eCollection 2024 Mar 15. iScience. 2024. PMID: 38439960 Free PMC article.
-
Notoginseng Leaf Triterpenes Ameliorates OGD/R-Induced Neuronal Injury via SIRT1/2/3-Foxo3a-MnSOD/PGC-1α Signaling Pathways Mediated by the NAMPT-NAD Pathway.Oxid Med Cell Longev. 2020 Oct 23;2020:7308386. doi: 10.1155/2020/7308386. eCollection 2020. Oxid Med Cell Longev. 2020. PMID: 33149812 Free PMC article.
References
-
- Schurr A. Energy metabolism, stress hormones and neural recovery from cerebral ischemia/hypoxia. Neurochem Int. 2002;41:1–8. - PubMed
-
- Belanger M, Allaman I, Magistretti PJ. Brain energy metabolism: Focus on astrocyte-neuron metabolic cooperation. Cell Metab. 2011;14:724–738. - PubMed
-
- Jaillard A, Cornu C, Durieux A, Moulin T, Boutitie F, Lees KR, et al. Hemorrhagic transformation in acute ischemic stroke. The mast-e study. Mast-e group. Stroke. 1999;30:1326–1332. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

