Macrophage function in tissue repair and remodeling requires IL-4 or IL-13 with apoptotic cells
- PMID: 28495875
- PMCID: PMC5556699
- DOI: 10.1126/science.aai8132
Macrophage function in tissue repair and remodeling requires IL-4 or IL-13 with apoptotic cells
Abstract
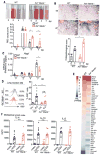
Tissue repair is a subset of a broad repertoire of interleukin-4 (IL-4)- and IL-13-dependent host responses during helminth infection. Here we show that IL-4 or IL-13 alone was not sufficient, but IL-4 or IL-13 together with apoptotic cells induced the tissue repair program in macrophages. Genetic ablation of sensors of apoptotic cells impaired the proliferation of tissue-resident macrophages and the induction of anti-inflammatory and tissue repair genes in the lungs after helminth infection or in the gut after induction of colitis. By contrast, the recognition of apoptotic cells was dispensable for cytokine-dependent induction of pattern recognition receptor, cell adhesion, or chemotaxis genes in macrophages. Detection of apoptotic cells can therefore spatially compartmentalize or prevent premature or ectopic activity of pleiotropic, soluble cytokines such as IL-4 or IL-13.
Copyright © 2017, American Association for the Advancement of Science.
Figures


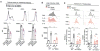
Comment in
-
Specific repair by discerning macrophages.Science. 2017 Jun 9;356(6342):1014. doi: 10.1126/science.aan6782. Science. 2017. PMID: 28596328 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

