Local amplifiers of IL-4Rα-mediated macrophage activation promote repair in lung and liver
- PMID: 28495878
- PMCID: PMC5737834
- DOI: 10.1126/science.aaj2067
Local amplifiers of IL-4Rα-mediated macrophage activation promote repair in lung and liver
Abstract
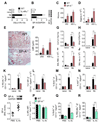
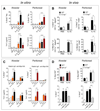
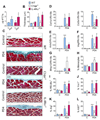
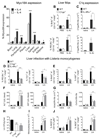
The type 2 immune response controls helminth infection and maintains tissue homeostasis but can lead to allergy and fibrosis if not adequately regulated. We have discovered local tissue-specific amplifiers of type 2-mediated macrophage activation. In the lung, surfactant protein A (SP-A) enhanced interleukin-4 (IL-4)-dependent macrophage proliferation and activation, accelerating parasite clearance and reducing pulmonary injury after infection with a lung-migrating helminth. In the peritoneal cavity and liver, C1q enhancement of type 2 macrophage activation was required for liver repair after bacterial infection, but resulted in fibrosis after peritoneal dialysis. IL-4 drives production of these structurally related defense collagens, SP-A and C1q, and the expression of their receptor, myosin 18A. These findings reveal the existence within different tissues of an amplification system needed for local type 2 responses.
Copyright © 2017, American Association for the Advancement of Science.
Conflict of interest statement
The authors declare no conflict of interest
Figures
Comment in
-
Specific repair by discerning macrophages.Science. 2017 Jun 9;356(6342):1014. doi: 10.1126/science.aan6782. Science. 2017. PMID: 28596328 No abstract available.
Similar articles
-
The lung environment controls alveolar macrophage metabolism and responsiveness in type 2 inflammation.Nat Immunol. 2019 May;20(5):571-580. doi: 10.1038/s41590-019-0352-y. Epub 2019 Apr 1. Nat Immunol. 2019. PMID: 30936493 Free PMC article.
-
Interleukin-4-promoted T helper 2 responses enhance Nippostrongylus brasiliensis-induced pulmonary pathology.Infect Immun. 2008 Dec;76(12):5535-42. doi: 10.1128/IAI.00210-08. Epub 2008 Sep 22. Infect Immun. 2008. PMID: 18809669 Free PMC article.
-
NK cells activated by Interleukin-4 in cooperation with Interleukin-15 exhibit distinctive characteristics.Proc Natl Acad Sci U S A. 2016 Sep 6;113(36):10139-44. doi: 10.1073/pnas.1600112113. Epub 2016 Aug 22. Proc Natl Acad Sci U S A. 2016. PMID: 27551096 Free PMC article.
-
Macrophage function in tissue repair and remodeling requires IL-4 or IL-13 with apoptotic cells.Science. 2017 Jun 9;356(6342):1072-1076. doi: 10.1126/science.aai8132. Epub 2017 May 11. Science. 2017. PMID: 28495875 Free PMC article.
-
Host protective roles of type 2 immunity: parasite killing and tissue repair, flip sides of the same coin.Semin Immunol. 2014 Aug;26(4):329-40. doi: 10.1016/j.smim.2014.06.003. Epub 2014 Jul 11. Semin Immunol. 2014. PMID: 25028340 Free PMC article. Review.
Cited by
-
Protective Effects of Aminooxyacetic Acid on Colitis Induced in Mice with Dextran Sulfate Sodium.Biomed Res Int. 2021 Dec 10;2021:1477345. doi: 10.1155/2021/1477345. eCollection 2021. Biomed Res Int. 2021. PMID: 35299827 Free PMC article.
-
The Role of Collectins and Galectins in Lung Innate Immune Defense.Front Immunol. 2018 Sep 4;9:1998. doi: 10.3389/fimmu.2018.01998. eCollection 2018. Front Immunol. 2018. PMID: 30233589 Free PMC article. Review.
-
Macrophage Heterogeneity in the Immunopathogenesis of Tuberculosis.Front Microbiol. 2018 May 23;9:1028. doi: 10.3389/fmicb.2018.01028. eCollection 2018. Front Microbiol. 2018. PMID: 29875747 Free PMC article. Review.
-
Increased levels of Gab1 and Gab2 adaptor proteins skew interleukin-4 (IL-4) signaling toward M2 macrophage-driven pulmonary fibrosis in mice.J Biol Chem. 2017 Aug 25;292(34):14003-14015. doi: 10.1074/jbc.M117.802066. Epub 2017 Jul 7. J Biol Chem. 2017. PMID: 28687632 Free PMC article.
-
Does tissue imprinting restrict macrophage plasticity?Nat Immunol. 2021 Feb;22(2):118-127. doi: 10.1038/s41590-020-00849-2. Epub 2021 Jan 18. Nat Immunol. 2021. PMID: 33462453 Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

