Endoplasmic reticulum (ER) Ca2+-channel activity contributes to ER stress and cone death in cyclic nucleotide-gated channel deficiency
- PMID: 28495882
- PMCID: PMC5500788
- DOI: 10.1074/jbc.M117.782326
Endoplasmic reticulum (ER) Ca2+-channel activity contributes to ER stress and cone death in cyclic nucleotide-gated channel deficiency
Abstract
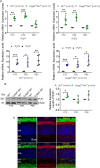
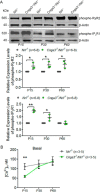
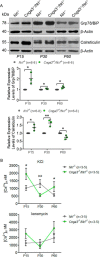
Endoplasmic reticulum (ER) stress and mislocalization of improperly folded proteins have been shown to contribute to photoreceptor death in models of inherited retinal degenerative diseases. In particular, mice with cone cyclic nucleotide-gated (CNG) channel deficiency, a model for achromatopsia, display both early-onset ER stress and opsin mistrafficking. By 2 weeks of age, these mice show elevated signaling from all three arms of the ER-stress pathway, and by 1 month, cone opsin is improperly distributed away from its normal outer segment location to other retinal layers. This work investigated the role of Ca2+-release channels in ER stress, protein mislocalization, and cone death in a mouse model of CNG-channel deficiency. We examined whether preservation of luminal Ca2+ stores through pharmacological and genetic suppression of ER Ca2+ efflux protects cones by attenuating ER stress. We demonstrated that the inhibition of ER Ca2+-efflux channels reduced all three arms of ER-stress signaling while improving opsin trafficking to cone outer segments and decreasing cone death by 20-35%. Cone-specific gene deletion of the inositol-1,4,5-trisphosphate receptor type I (IP3R1) also significantly increased cone density in the CNG-channel-deficient mice, suggesting that IP3R1 signaling contributes to Ca2+ homeostasis and cone survival. Consistent with the important contribution of organellar Ca2+ signaling in this achromatopsia mouse model, significant differences in dynamic intraorganellar Ca2+ levels were detected in CNG-channel-deficient cones. These results thus identify a novel molecular link between Ca2+ homeostasis and cone degeneration, thereby revealing novel therapeutic targets to preserve cones in inherited retinal degenerative diseases.
Keywords: CNG channel; calcium channel; endoplasmic reticulum stress (ER stress); inositol trisphosphate receptor (InsP3R); photoreceptor; ryanodine receptor.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures





Similar articles
-
Preservation of endoplasmic reticulum (ER) Ca2+ stores by deletion of inositol-1,4,5-trisphosphate receptor type 1 promotes ER retrotranslocation, proteostasis, and protein outer segment localization in cyclic nucleotide-gated channel-deficient cone photoreceptors.FASEB J. 2021 Jun;35(6):e21579. doi: 10.1096/fj.202002711R. FASEB J. 2021. PMID: 33960001 Free PMC article.
-
cGMP/Protein Kinase G Signaling Suppresses Inositol 1,4,5-Trisphosphate Receptor Phosphorylation and Promotes Endoplasmic Reticulum Stress in Photoreceptors of Cyclic Nucleotide-gated Channel-deficient Mice.J Biol Chem. 2015 Aug 21;290(34):20880-20892. doi: 10.1074/jbc.M115.641159. Epub 2015 Jun 29. J Biol Chem. 2015. PMID: 26124274 Free PMC article.
-
Promotion of endoplasmic reticulum retrotranslocation by overexpression of E3 ubiquitin-protein ligase synoviolin 1 reduces endoplasmic reticulum stress and preserves cone photoreceptors in cyclic nucleotide-gated channel deficiency.FASEB J. 2024 Sep 15;38(17):e70021. doi: 10.1096/fj.202400198R. FASEB J. 2024. PMID: 39215566
-
Inositol 1,4,5-trisphosphate receptor-isoform diversity in cell death and survival.Biochim Biophys Acta. 2014 Oct;1843(10):2164-83. doi: 10.1016/j.bbamcr.2014.03.007. Epub 2014 Mar 15. Biochim Biophys Acta. 2014. PMID: 24642269 Review.
-
Inositol 1,4,5-trisphosphate receptors in the endoplasmic reticulum: A single-channel point of view.Cell Calcium. 2015 Jul;58(1):67-78. doi: 10.1016/j.ceca.2014.12.008. Epub 2014 Dec 18. Cell Calcium. 2015. PMID: 25555684 Free PMC article. Review.
Cited by
-
Primary and Secondary Cone Cell Death Mechanisms in Inherited Retinal Diseases and Potential Treatment Options.Int J Mol Sci. 2022 Jan 10;23(2):726. doi: 10.3390/ijms23020726. Int J Mol Sci. 2022. PMID: 35054919 Free PMC article. Review.
-
Ryanodine Receptor 2 Contributes to Impaired Protein Localization in Cyclic Nucleotide-Gated Channel Deficiency.eNeuro. 2019 Jun 27;6(3):ENEURO.0119-19.2019. doi: 10.1523/ENEURO.0119-19.2019. Print 2019 May/Jun. eNeuro. 2019. PMID: 31182474 Free PMC article.
-
Marchantia polymorpha L. ethanol extract induces apoptosis in hepatocellular carcinoma cells via intrinsic- and endoplasmic reticulum stress-associated pathways.Chin Med. 2021 Sep 28;16(1):94. doi: 10.1186/s13020-021-00504-4. Chin Med. 2021. PMID: 34583719 Free PMC article.
-
The cGMP-Dependent Protein Kinase 2 Contributes to Cone Photoreceptor Degeneration in the Cnga3-Deficient Mouse Model of Achromatopsia.Int J Mol Sci. 2020 Dec 23;22(1):52. doi: 10.3390/ijms22010052. Int J Mol Sci. 2020. PMID: 33374621 Free PMC article.
-
PSMD4 regulates the malignancy of esophageal cancer cells by suppressing endoplasmic reticulum stress.Kaohsiung J Med Sci. 2019 Oct;35(10):591-597. doi: 10.1002/kjm2.12093. Epub 2019 Jun 4. Kaohsiung J Med Sci. 2019. PMID: 31162820 Free PMC article.
References
-
- Kaupp U. B., and Seifert R. (2002) Cyclic nucleotide-gated ion channels. Physiol. Rev. 82, 769–824 - PubMed
-
- Kohl S., Varsanyi B., Antunes G. A., Baumann B., Hoyng C. B., Jägle H., Rosenberg T., Kellner U., Lorenz B., Salati R., Jurklies B., Farkas A., Andreasson S., Weleber R. G., Jacobson S. G., et al. (2005) CNGB3 mutations account for 50% of all cases with autosomal recessive achromatopsia. Eur. J. Hum. Genet. 13, 302–308 - PubMed
-
- Mears A. J., Kondo M., Swain P. K., Takada Y., Bush R. A., Saunders T. L., Sieving P. A., and Swaroop A. (2001) Nrl is required for rod photoreceptor development. Nat. Genet. 29, 447–452 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

