Intratumoral Injection of HSV1716, an Oncolytic Herpes Virus, Is Safe and Shows Evidence of Immune Response and Viral Replication in Young Cancer Patients
- PMID: 28495911
- PMCID: PMC10546618
- DOI: 10.1158/1078-0432.CCR-16-2900
Intratumoral Injection of HSV1716, an Oncolytic Herpes Virus, Is Safe and Shows Evidence of Immune Response and Viral Replication in Young Cancer Patients
Abstract
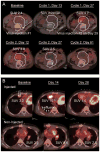
Purpose: HSV1716 is an oncolytic herpes simplex virus-1 (HSV-1) studied in adults via injection into the brain and superficial tumors. To determine the safety of administering HSV1716 to pediatric patients with cancer, we conducted a phase I trial of image-guided injection in young patients with relapsed or refractory extracranial cancers.Experimental Design: We delivered a single dose of 105 to 107 infectious units of HSV1716 via computed tomography-guided intratumoral injection and measured tumor responses by imaging. Patients were eligible for up to three more doses if they achieved stable disease. We monitored HSV-1 serum titers and shedding by PCR and culture.Results: We administered a single dose of HSV1716 to eight patients and two doses to one patient. We did not observe any dose-limiting toxicities. Adverse events attributed to virus included low-grade fever, chills, and mild cytopenias. Six of eight HSV-1 seronegative patients at baseline showed seroconversion on day 28. Six of nine patients had detectable HSV-1 genomes by PCR in peripheral blood appearing on day +4 consistent with de novo virus replication. Two patients had transient focal increases in metabolic activity on 18fluorine-deoxyglucose PET, consistent with inflammatory reactions. In one case, the same geographic region that flared later appeared necrotic on imaging. No patient had an objective response to HSV1716.Conclusions: Intratumoral HSV1716 is safe and well-tolerated without shedding in children and young adults with late-stage, aggressive cancer. Viremia consistent with virus replication and transient inflammatory reactions hold promise for future HSV1716 studies. Clin Cancer Res; 23(14); 3566-74. ©2017 AACR.
©2017 American Association for Cancer Research.
Figures
Similar articles
-
First-in-Human Intravenous Seprehvir in Young Cancer Patients: A Phase 1 Clinical Trial.Mol Ther. 2019 Nov 6;27(11):1930-1938. doi: 10.1016/j.ymthe.2019.08.020. Epub 2019 Sep 10. Mol Ther. 2019. PMID: 31570234 Free PMC article. Clinical Trial.
-
Expression of inhibitor of growth 4 by HSV1716 improves oncolytic potency and enhances efficacy.Cancer Gene Ther. 2012 Jul;19(7):499-507. doi: 10.1038/cgt.2012.24. Epub 2012 May 18. Cancer Gene Ther. 2012. PMID: 22595793
-
Potential for efficacy of the oncolytic Herpes simplex virus 1716 in patients with oral squamous cell carcinoma.Head Neck. 2008 Aug;30(8):1045-51. doi: 10.1002/hed.20840. Head Neck. 2008. PMID: 18615711 Clinical Trial.
-
Oncolytic virus therapy using genetically engineered herpes simplex viruses.Front Biosci. 2008 Jan 1;13:2060-4. doi: 10.2741/2823. Front Biosci. 2008. PMID: 17981691 Review.
-
Oncolytic Virotherapy by HSV.Adv Exp Med Biol. 2018;1045:63-84. doi: 10.1007/978-981-10-7230-7_4. Adv Exp Med Biol. 2018. PMID: 29896663 Review.
Cited by
-
Current clinical landscape of oncolytic viruses as novel cancer immunotherapeutic and recent preclinical advancements.Front Immunol. 2022 Aug 25;13:953410. doi: 10.3389/fimmu.2022.953410. eCollection 2022. Front Immunol. 2022. PMID: 36091031 Free PMC article. Review.
-
Biological Characterization and Therapeutics for Subscalp Recurrent in Intracranial Glioblastoma.Onco Targets Ther. 2020 Sep 11;13:9085-9099. doi: 10.2147/OTT.S265322. eCollection 2020. Onco Targets Ther. 2020. PMID: 32982297 Free PMC article.
-
Biological treatment of pediatric sarcomas by combined virotherapy and NK cell therapy.BMC Cancer. 2019 Dec 3;19(1):1172. doi: 10.1186/s12885-019-6387-5. BMC Cancer. 2019. PMID: 31795974 Free PMC article.
-
Oncolytic virotherapy evolved into the fourth generation as tumor immunotherapy.J Transl Med. 2023 Jul 25;21(1):500. doi: 10.1186/s12967-023-04360-8. J Transl Med. 2023. PMID: 37491263 Free PMC article. Review.
-
Trabectedin promotes oncolytic virus antitumor efficacy, viral gene expression, and immune effector function in models of bone sarcoma.Mol Ther Oncol. 2024 Sep 26;32(4):200886. doi: 10.1016/j.omton.2024.200886. eCollection 2024 Dec 19. Mol Ther Oncol. 2024. PMID: 39492947 Free PMC article.
References
-
- Perry LJ, McGeoch DJ. The DNA sequences of the long repeat region and adjoining parts of the long unique region in the genome of herpes simplex virus type 1. J Gen Virol. 1988;69(Pt 11):2831–46. - PubMed
-
- McGeoch DJ, Dalrymple MA, Davison AJ, Dolan A, Frame MC, McNab D, et al. The complete DNA sequence of the long unique region in the genome of herpes simplex virus type 1. J Gen Virol. 1988;69(Pt 7):1531–74. - PubMed
-
- Whitley R, Gnann J. The epidemiology and clinical manifestation sof herpes simplex virus infections. In: Lopez C, editor. The Human Herpesviruses. New York: Raven Press; 1993. pp. 69–105.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

