Maternal Torso-Like Coordinates Tissue Folding During Drosophila Gastrulation
- PMID: 28495958
- PMCID: PMC5500143
- DOI: 10.1534/genetics.117.200576
Maternal Torso-Like Coordinates Tissue Folding During Drosophila Gastrulation
Abstract
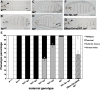
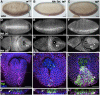
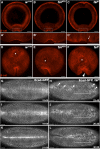
The rapid and orderly folding of epithelial tissue during developmental processes such as gastrulation requires the precise coordination of changes in cell shape. Here, we report that the perforin-like protein Torso-like (Tsl), the key extracellular determinant for Drosophila embryonic terminal patterning, also functions to control epithelial morphogenesis. We find that tsl null mutants display a ventral cuticular hole phenotype that is independent of the loss of terminal structures, and arises as a consequence of mesoderm invagination defects. We show that the holes are caused by uncoordinated constriction of ventral cell apices, resulting in the formation of an incomplete ventral furrow. Consistent with these data, we find that loss of tsl is sensitive to gene dosage of RhoGEF2, a critical mediator of Rho1-dependent ventral cell shape changes during furrow formation, suggesting that Tsl may act in this pathway. In addition, loss of tsl strongly suppressed the effects of ectopic expression of Folded Gastrulation (Fog), a secreted protein that promotes apical constriction. Taken together, our data suggest that Tsl controls Rho1-mediated apical constriction via Fog. Therefore, we propose that Tsl regulates extracellular Fog activity to synchronize cell shape changes and coordinate ventral morphogenesis in Drosophila Identifying the Tsl-mediated event that is common to both terminal patterning and morphogenesis will be valuable for our understanding of the extracellular control of developmental signaling by perforin-like proteins.
Keywords: Drosophila; MACPF; Torso-like; gastrulation; morphogenesis.
Copyright © 2017 by the Genetics Society of America.
Figures

Similar articles
-
Abelson kinase (Abl) and RhoGEF2 regulate actin organization during cell constriction in Drosophila.Development. 2007 Feb;134(3):567-78. doi: 10.1242/dev.02748. Epub 2007 Jan 3. Development. 2007. PMID: 17202187
-
Rho1 activation recapitulates early gastrulation events in the ventral, but not dorsal, epithelium of Drosophila embryos.Elife. 2020 Nov 17;9:e56893. doi: 10.7554/eLife.56893. Elife. 2020. PMID: 33200987 Free PMC article.
-
Hyperactivation of the folded gastrulation pathway induces specific cell shape changes.Development. 1998 Feb;125(4):589-97. doi: 10.1242/dev.125.4.589. Development. 1998. PMID: 9435280
-
The Fog signaling pathway: insights into signaling in morphogenesis.Dev Biol. 2014 Oct 1;394(1):6-14. doi: 10.1016/j.ydbio.2014.08.003. Epub 2014 Aug 12. Dev Biol. 2014. PMID: 25127992 Free PMC article. Review.
-
MACPF/CDC proteins in development: Insights from Drosophila torso-like.Semin Cell Dev Biol. 2017 Dec;72:163-170. doi: 10.1016/j.semcdb.2017.05.003. Epub 2017 May 12. Semin Cell Dev Biol. 2017. PMID: 28506893 Review.
Cited by
-
Optogenetic Rescue of a Patterning Mutant.Curr Biol. 2020 Sep 7;30(17):3414-3424.e3. doi: 10.1016/j.cub.2020.06.059. Epub 2020 Jul 23. Curr Biol. 2020. PMID: 32707057 Free PMC article.
-
Small GTPases modulate intrinsic and extrinsic forces that control epithelial folding in Drosophila embryos.Small GTPases. 2021 Sep-Nov;12(5-6):416-428. doi: 10.1080/21541248.2021.1926879. Epub 2021 Jun 28. Small GTPases. 2021. PMID: 33985411 Free PMC article. Review.
-
Insulin-Like Signalling Influences the Coordination of Larval Hemocyte Number with Body Size in Drosophila melanogaster.G3 (Bethesda). 2020 Jul 7;10(7):2213-2220. doi: 10.1534/g3.120.401313. G3 (Bethesda). 2020. PMID: 32341056 Free PMC article.
-
Finishing the egg.Genetics. 2024 Jan 3;226(1):iyad183. doi: 10.1093/genetics/iyad183. Genetics. 2024. PMID: 38000906 Free PMC article.
-
The torso-like gene functions to maintain the structure of the vitelline membrane in Nasonia vitripennis, implying its co-option into Drosophila axis formation.Biol Open. 2019 Sep 25;8(9):bio046284. doi: 10.1242/bio.046284. Biol Open. 2019. PMID: 31488408 Free PMC article.
References
-
- Barrett K., Leptin M., Settleman J., 1997. The Rho GTPase and a putative RhoGEF mediate a signaling pathway for the cell shape changes in Drosophila gastrulation. Cell 91: 905–915. - PubMed
-
- Casanova J., Struhl G., 1989. Localized surface activity of torso, a receptor tyrosine kinase, specifies terminal body pattern in Drosophila. Genes Dev. 3: 2025–2038. - PubMed
-
- Costa M., Wilson E. T., Wieschaus E., 1994. A putative cell signal encoded by the folded gastrulation gene coordinates cell shape changes during Drosophila gastrulation. Cell 76: 1075–1089. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

