Peiminine inhibits colorectal cancer cell proliferation by inducing apoptosis and autophagy and modulating key metabolic pathways
- PMID: 28496003
- PMCID: PMC5564592
- DOI: 10.18632/oncotarget.17411
Peiminine inhibits colorectal cancer cell proliferation by inducing apoptosis and autophagy and modulating key metabolic pathways
Abstract
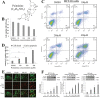
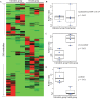
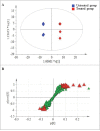
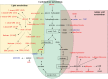
Peiminine, a compound extracted from the bulbs of Fritillaria thunbergii and traditionally used as a medication in China and other Asian countries, was reported to inhibit colorectal cancer cell proliferation and tumor growth by inducing autophagic cell death. However, its mechanism of anticancer action is not well understood, especially at the metabolic level, which was thought to primarily account for peiminine's efficacy against cancer. Using an established metabolomic profiling platform combining ultra-performance liquid chromatography/tandem mass spectrometry with gas chromatography/mass spectrometry, we identified metabolic alterations in colorectal cancer cell line HCT-116 after peiminine treatment. Among the identified 236 metabolites, the levels of 57 of them were significantly (p < 0.05) different between peiminine-treated and -untreated cells in which 45 metabolites were increased and the other 12 metabolites were decreased. Several of the affected metabolites, including glucose, glutamine, oleate (18:1n9), and lignocerate (24:0), may be involved in regulation of the phosphoinositide 3-kinase/Akt/mammalian target of rapamycin (mTOR) pathway and in the oxidative stress response upon peiminine exposure. Peiminine predominantly modulated the pathways responsible for metabolism of amino acids, carbohydrates, and lipids. Collectively, these results provide new insights into the mechanisms by which peiminine modulates metabolic pathways to inhibit colorectal cancer cell growth, supporting further exploration of peiminine as a potential new strategy for treating colorectal cancer.
Keywords: cancer therapy; colorectal cancer; metabolomics; natural product; peiminine.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Li Q, Wei P, Huang B, Xu Y, Li X, Li Y, Cai S, Li D. MAEL expression links epithelial-mesenchymal transition and stem cell properties in colorectal cancer. Int J Cancer. 2016;139:2502–2511. - PubMed
-
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. - PubMed
-
- Xue Y, Gu D, Ma G, Zhu L, Hua Q, Chu H, Tong N, Chen J, Zhang Z, Wang M. Genetic variants in lncRNA HOTAIR are associated with risk of colorectal cancer. Mutagenesis. 2015;30:303–310. - PubMed
-
- Aran V, Victorino AP, Thuler LC, Ferreira CG. Colorectal Cancer: Epidemiology, Disease Mechanisms and Interventions to Reduce Onset and Mortality. Clin Colorectal Cancer. 2016;15:195–203. - PubMed
-
- Coghlin C, Murray GI. Biomarkers of colorectal cancer: recent advances and future challenges. Proteomics Clin Appl. 2015;9:64–71. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

