Days weaving the lagging strand synthesis of DNA - A personal recollection of the discovery of Okazaki fragments and studies on discontinuous replication mechanism
- PMID: 28496054
- PMCID: PMC5489436
- DOI: 10.2183/pjab.93.020
Days weaving the lagging strand synthesis of DNA - A personal recollection of the discovery of Okazaki fragments and studies on discontinuous replication mechanism
Abstract
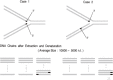
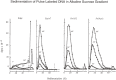
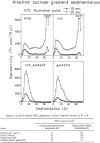
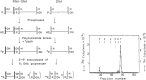
At DNA replication forks, the overall growth of the antiparallel two daughter DNA chains appears to occur 5'-to-3' direction in the leading-strand and 3'-to-5' direction in the lagging-strand using enzyme system only able to elongate 5'-to-3' direction, and I describe in this review how we have analyzed and proved the lagging strand multistep synthesis reactions, called Discontinuous Replication Mechanism, which involve short RNA primer synthesis, primer-dependent short DNA chains (Okazaki fragments) synthesis, primer removal from the Okazaki fragments and gap filling between Okazaki fragments by RNase H and DNA polymerase I, and long lagging strand formation by joining between Okazaki fragments with DNA ligase.
Keywords: DNA ligase; Okazaki fragments; function of RNase H and DNA polymerase 1; lagging strand synthesis; primer RNA dependent synthesis of Okazaki fragments; processing of Okazaki fragments before ligation.
Figures







Similar articles
-
RNA primer-primase complexes serve as the signal for polymerase recycling and Okazaki fragment initiation in T4 phage DNA replication.Proc Natl Acad Sci U S A. 2017 May 30;114(22):5635-5640. doi: 10.1073/pnas.1620459114. Epub 2017 May 15. Proc Natl Acad Sci U S A. 2017. PMID: 28507156 Free PMC article.
-
Primer release is the rate-limiting event in lagging-strand synthesis mediated by the T7 replisome.Proc Natl Acad Sci U S A. 2016 May 24;113(21):5916-21. doi: 10.1073/pnas.1604894113. Epub 2016 May 9. Proc Natl Acad Sci U S A. 2016. PMID: 27162371 Free PMC article.
-
The control mechanism for lagging strand polymerase recycling during bacteriophage T4 DNA replication.Mol Cell. 2006 Jan 20;21(2):153-64. doi: 10.1016/j.molcel.2005.11.029. Mol Cell. 2006. PMID: 16427006
-
Repetitive lagging strand DNA synthesis by the bacteriophage T4 replisome.Mol Biosyst. 2008 Nov;4(11):1070-4. doi: 10.1039/b812163j. Epub 2008 Sep 29. Mol Biosyst. 2008. PMID: 18931782 Review.
-
Eukaryotic DNA replication. Enzymes and proteins acting at the fork.Eur J Biochem. 1990 Dec 27;194(3):699-712. doi: 10.1111/j.1432-1033.1990.tb19460.x. Eur J Biochem. 1990. PMID: 2269294 Review.
Cited by
-
Solution to the 50-year-old Okazaki-fragment problem.Proc Natl Acad Sci U S A. 2019 Feb 26;116(9):3358-3360. doi: 10.1073/pnas.1900372116. Epub 2019 Feb 15. Proc Natl Acad Sci U S A. 2019. PMID: 30770440 Free PMC article. No abstract available.
-
Near-continuously synthesized leading strands in Escherichia coli are broken by ribonucleotide excision.Proc Natl Acad Sci U S A. 2019 Jan 22;116(4):1251-1260. doi: 10.1073/pnas.1814512116. Epub 2019 Jan 7. Proc Natl Acad Sci U S A. 2019. PMID: 30617079 Free PMC article.
-
Molecular mechanisms of eukaryotic origin initiation, replication fork progression, and chromatin maintenance.Biochem J. 2020 Sep 30;477(18):3499-3525. doi: 10.1042/BCJ20200065. Biochem J. 2020. PMID: 32970141 Free PMC article.
-
Dinoroseobacter shibae Outer Membrane Vesicles Are Enriched for the Chromosome Dimer Resolution Site dif.mSystems. 2021 Jan 12;6(1):e00693-20. doi: 10.1128/mSystems.00693-20. mSystems. 2021. PMID: 33436507 Free PMC article.
-
Genome-Wide Profiling of Endogenous Single-Stranded DNA Using the SSiNGLe-P1 Method.Int J Mol Sci. 2023 Jul 27;24(15):12062. doi: 10.3390/ijms241512062. Int J Mol Sci. 2023. PMID: 37569439 Free PMC article.
References
-
- Watson J., Crick F. (1953) Molecular structure of nucleic acids. Nature 171, 737–738. - PubMed
-
- Lehman I.R., Bessman M.J., Simms E.S., Kornberg A. (1958) Enzymatic synthesis of deoxyribonucleic acid. 1 Preparation of substrates and partial purification of an enzyme from Escherichia coli. J. Biol. Chem. 233, 163–170. - PubMed
-
- Okazaki R., Okazaki T., Kuriki Y. (1960) Isolation of thymidine diphosphate rhamnose and a novel thymidine diphosphate sugar compound from Escherichia coli strain B. Biochim. Biophys. Acta 38, 384–386. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

