Isolated Bacillus subtilis strain 330-2 and its antagonistic genes identified by the removing PCR
- PMID: 28496135
- PMCID: PMC5431837
- DOI: 10.1038/s41598-017-01940-9
Isolated Bacillus subtilis strain 330-2 and its antagonistic genes identified by the removing PCR
Abstract
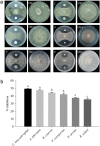
Plant growth-promoting bacteria (PGPB) may trigger tolerance against biotic/abiotic stresses and growth enhancement in plants. In this study, an endophytic bacterial strain from rapeseed was isolated to assess its role in enhancing plant growth and tolerance to abiotic stresses, as well as banded leaf and sheath blight disease in maize. Based on 16S rDNA and BIOLOG test analysis, the 330-2 strain was identified as Bacillus subtilis. The strain produced indole-3-acetic acid, siderophores, lytic enzymes and solubilized different sources of organic/inorganic phosphates and zinc. Furthermore, the strain strongly suppressed the in vitro growth of Rhizoctonia solani AG1-IA, Botrytis cinerea, Fusarium oxysporum, Alternaria alternata, Cochliobolus heterostrophus, and Nigrospora oryzae. The strain also significantly increased the seedling growth (ranging 14-37%) of rice and maize. Removing PCR analysis indicated that 114 genes were differentially expressed, among which 10%, 32% and 10% were involved in antibiotic production (e.g., srfAA, bae, fen, mln, and dfnI), metabolism (e.g., gltA, pabA, and ggt) and transportation of nutrients (e.g., fhu, glpT, and gltT), respectively. In summary, these results clearly indicate the effectiveness and mechanisms of B. subtilis strain 330-2 in enhancing plant growth, as well as tolerance to biotic/abiotic stresses, which suggests that the strain has great potential for commercialization as a vital biological control agent.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures






References
-
- Bashan Y, Holguin G. Proposal for the division of plant growth-promoting rhizobacteria into two classifications: biocontrol-PGPB (plant growth-promoting bacteria) and PGPB. Soil Biol. Biochem. 1998;30:1225–1228. doi: 10.1016/S0038-0717(97)00187-9. - DOI
-
- Compant S, Clément C, Sessitsch A. Plant growth-promoting bacteria in the rhizo- and endosphere of plants: Their role, colonization, mechanisms involved and prospects for utilization. Soil Biol. Biochem. 2010;42:669–678. doi: 10.1016/j.soilbio.2009.11.024. - DOI
-
- Yao AV, et al. Effect of FZB 24® Bacillus subtilis as a biofertilizer on cotton yields in field tests. Arch. Phytopathol. Plant Pro. 2006;39:323–328. doi: 10.1080/03235400600655347. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

