Measurement of local temperature increments induced by cultured HepG2 cells with micro-thermocouples in a thermally stabilized system
- PMID: 28496166
- PMCID: PMC5431931
- DOI: 10.1038/s41598-017-01891-1
Measurement of local temperature increments induced by cultured HepG2 cells with micro-thermocouples in a thermally stabilized system
Abstract
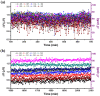
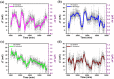
To monitor the temperature distribution of a cell and its changes under varied conditions is currently a technical challenge. A variety of non-contact methods used for measuring cellular temperature have been developed, where changes of local temperature at cell-level and sub-cell-level are indirectly calculated through the changes in intensity, band-shape, bandwidth, lifetime or polarization anisotropy of the fluorescence spectra recorded from the nano-sized fluorescent materials pre-injected into the target cell. Unfortunately, the optical properties of the fluorescent nano-materials may be affected by complicated intracellular environment, leading to unexpected measurement errors and controversial arguments. Here, we attempted to offer an alternative approach for measuring the absolute increments of local temperature in micro-Testing Zones induced by live cells. In this method, built-in high-performance micro-thermocouple arrays and double-stabilized system with a stability of 10 mK were applied. Increments of local temperature close to adherent human hepatoblastoma (HepG2) cells were continuously recorded for days without stimulus, showing frequent fluctuations within 60 mK and a maximum increment by 285 mK. This method may open a door for real-time recording of the absolute local temperature increments of individual cells, therefore offering valuable information for cell biology and clinical therapy in the field of cancer research.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures






References
-
- Lowell BB, Spiegelman BM. Towards a molecular understanding of adaptive thermogenesis. Nature. 2000;404:652–660. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

