Efficacy and safety of thiazolidinediones in diabetes patients with renal impairment: a systematic review and meta-analysis
- PMID: 28496176
- PMCID: PMC5431943
- DOI: 10.1038/s41598-017-01965-0
Efficacy and safety of thiazolidinediones in diabetes patients with renal impairment: a systematic review and meta-analysis
Abstract
We conducted a systematic review and meta-analysis to evaluate the efficacy and safety of TZDs in treatment of diabetes mellitus patients with renal impairment. We searched PubMed, EMBASE and Cochrane Central Register of Controlled Trials. Randomized controlled trials (RCTs), cohort studies, and case-control studies that investigated the effects of TZDs in patients with diabetes and renal impairment were eligible. Outcomes included glycosylated hemoglobin, fasting plasma glucose, serum lipids, and patient-important outcomes (i.e. hypoglycemia, weight, edema, cardiovascular events and mortality). 19 RCTs and 3 cohort studies involving 21,803 patients with diabetes and renal impairment were included. Meta-analysis of RCTs showed that TZDs could significantly reduce HbA1c (MD -0.64, 95%CI -0.93 to -0.35), FPG (MD -26.27, 95%CI -44.90 to -7.64) and increase HDL levels (MD 3.70, 95%CI 1.10, 6.29). TZDs could increase weight (MD 3.23, 95% CI 2.29 to 4.16) and risk of edema (RR 2.96, 95% CI 1.22 to 7.20). Their effects on risk of hypoglycemia (RR 1.46, 95% CI 0.65 to 3.29), heart failure (RR 0.64, 95% CI 0.15 to 2.66), angina (RR 1.45, 95% CI 0.23 to 8.95) and all-cause mortality (RR 0.40, 95% CI 0.08 to 2.01) are uncertain. Results from cohort studies were similar to RCTs.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
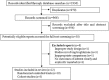
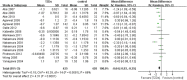


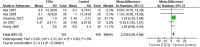

References
-
- IDF DIABETES ATLAS. http://www.diabetesatlas.org/.
-
- National Kidney Foundation. KDOQI Clinical Practice Guideline for Diabetes and CKD: 2012 Update. Am J Kidney Dis60, 850–886, doi:10.1053/j.ajkd.2012.07.005 (2012). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

