'Gardos Channelopathy': a variant of hereditary Stomatocytosis with complex molecular regulation
- PMID: 28496185
- PMCID: PMC5431847
- DOI: 10.1038/s41598-017-01591-w
'Gardos Channelopathy': a variant of hereditary Stomatocytosis with complex molecular regulation
Abstract
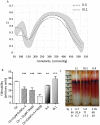
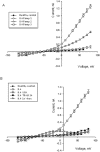
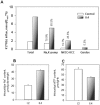
The Gardos channel is a Ca2+ sensitive, K+ selective channel present in several tissues including RBCs, where it is involved in cell volume regulation. Recently, mutations at two different aminoacid residues in KCNN4 have been reported in patients with hereditary xerocytosis. We identified by whole exome sequencing a new family with two members affected by chronic hemolytic anemia carrying mutation R352H in the KCNN4 gene. No additional mutations in genes encoding for RBCs cytoskeletal, membrane or channel proteins were detected. We performed functional studies on patients' RBCs to evaluate the effects of R352H mutation on the cellular properties and eventually on the clinical phenotype. Gardos channel hyperactivation was demonstrated in circulating erythrocytes and erythroblasts differentiated ex-vivo from peripheral CD34+ cells. Pathological alterations in the function of multiple ion transport systems were observed, suggesting the presence of compensatory effects ultimately preventing cellular dehydration in patient's RBCs; moreover, flow cytometry and confocal fluorescence live-cell imaging showed Ca2+ overload in the RBCs of both patients and hypersensitivity of Ca2+ uptake by RBCs to swelling. Altogether these findings suggest that the 'Gardos channelopathy' is a complex pathology, to some extent different from the common hereditary xerocytosis.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

