Upregulation and biological function of transmembrane protein 119 in osteosarcoma
- PMID: 28496199
- PMCID: PMC5454443
- DOI: 10.1038/emm.2017.41
Upregulation and biological function of transmembrane protein 119 in osteosarcoma
Abstract
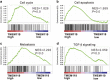
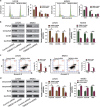
Osteosarcoma is suggested to be caused by genetic and molecular alterations that disrupt osteoblast differentiation. Recent studies have reported that transmembrane protein 119 (TMEM119) contributes to osteoblast differentiation and bone development. However, the level of TMEM119 expression and its roles in osteosarcoma have not yet been elucidated. In the present study, TMEM119 mRNA and protein expression was found to be up-regulated in osteosarcoma compared with normal bone cyst tissues. The level of TMEM119 protein expression was strongly associated with tumor size, clinical stage, distant metastasis and overall survival time. Moreover, gene set enrichment analysis (GSEA) of the Gene Expression Omnibus (GEO) GSE42352 dataset revealed TMEM119 expression in osteosarcoma tissues to be positively correlated with cell cycle, apoptosis, metastasis and TGF-β signaling. We then knocked down TMEM119 expression in U2OS and MG63 cells using small interfering RNA, which revealed that downregulation of TMEM119 could inhibit the proliferation of osteosarcoma cells by inducing cell cycle arrest in G0/G1 phase and apoptosis. We also found that TMEM119 knockdown significantly inhibited cell migration and invasion, and decreased the expression of TGF-β pathway-related factors (BMP2, BMP7 and TGF-β). TGF-β application rescued the inhibitory effects of TMEM119 knockdown on osteosarcoma cell migration and invasion. Further in vitro experiments with a TGF-β inhibitor (SB431542) or BMP inhibitor (dorsomorphin) suggested that TMEM119 significantly promotes cell migration and invasion, partly through TGF-β/BMP signaling. In conclusion, our data support the notion that TMEM119 contributes to the proliferation, migration and invasion of osteosarcoma cells, and functions as an oncogene in osteosarcoma.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Gill J, Ahluwalia MK, Geller D, Gorlick R. New targets and approaches in osteosarcoma. Pharmacol Ther 2013; 137: 89–99. - PubMed
-
- Ottaviani G, Jaffe N. The epidemiology of osteosarcoma. Cancer Treat Res 2009; 152: 3–13. - PubMed
-
- Haydon RC, Luu HH, He TC. Osteosarcoma and osteoblastic differentiation: a new perspective on oncogenesis. Clin Orthop Relat Res 2007; 454: 237–246. - PubMed
-
- Thomas D, Kansara M. Epigenetic modifications in osteogenic differentiation and transformation. J Cell Biochem 2006; 98: 757–769. - PubMed
-
- Tan ML, Choong PF, Dass CR. Osteosarcoma: conventional treatment vs. gene therapy. Cancer Biol Ther 2009; 8: 106–117. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

