Development of a potent invigorator of immune responses endowed with both preventive and therapeutic properties
- PMID: 28496303
- PMCID: PMC5422320
- DOI: 10.2147/BTT.S128308
Development of a potent invigorator of immune responses endowed with both preventive and therapeutic properties
Abstract
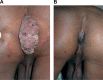
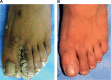
This article reviews briefly the making of an immunoprophylactic-cum-immunotherapeutic vaccine against leprosy. The vaccine is based on cultivable, heat-killed atypical mycobacteria, whose gene sequence is now known. It has been named Mycobacterium indicus pranii. It has received the approval of the Drug Controller General of India and the US Food and Drug Administration. Besides leprosy, M. indicus pranii has found utility in the treatment of category II ("difficult to treat") tuberculosis. It also heals ugly anogenital warts. It has preventive and therapeutic action against SP2/O myelomas. It is proving to be a potent adjuvant for enhancing antibody titers of a recombinant vaccine against human chorionic gonadotropin, with the potential of preventing pregnancy without derangement of ovulation and menstrual regularity in sexually active women.
Keywords: adjuvant; anogenital warts; leprosy; myeloma; tuberculosis.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures





References
-
- Talwar GP. An immunotherapeutic vaccine for multibacillary leprosy. Int Rev Immunol. 1999;18:229–249. - PubMed
-
- Talwar GP, Krishnan AD, Jha P, Mehra V. Intracellular growth of an obligatory parasite Mycobacterium leprae: host bacterial interactions. Biochimie. 1974;56:231–237. - PubMed
-
- Mustafa AS, Talwar GP. Five cultivable mycobacterial strains giving blast transformation and leukocyte migration inhibition of leukocytes analogous to Mycobacterium leprae. Lepr India. 1978;50:498–508. - PubMed
-
- Mustafa AS, Talwar GP. Early and late reactions in tuberculoid and lepromatous leprosy patients with lepromins from Mycobacterium leprae and five selected cultivable mycobacteria. Lepr India. 1978;50:566–571. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

