The effect of indacaterol/glycopyrronium versus tiotropium or salmeterol/fluticasone on the prevention of clinically important deterioration in COPD
- PMID: 28496316
- PMCID: PMC5422319
- DOI: 10.2147/COPD.S133307
The effect of indacaterol/glycopyrronium versus tiotropium or salmeterol/fluticasone on the prevention of clinically important deterioration in COPD
Abstract
Background: Endpoints that evaluate deterioration rather than improvement of disease may have clinical utility in COPD. In this analysis, we compared the effects of different maintenance treatments on the prevention of clinically important deterioration (CID) in moderate-to-severe COPD patients.
Methods: Data were analyzed from three 26-week studies comparing indacaterol/glycopyrronium (IND/GLY) with tiotropium (TIO) or salmeterol/fluticasone (SFC). Two definitions of CID were used; each was a composite of three outcome measures typically associated with COPD. Definition 1 (D1) comprised a ≥100 mL decrease in forced expiratory volume in 1 second (FEV1), a ≥4-unit increase in St George's Respiratory Questionnaire, and a moderate-to-severe COPD exacerbation. In Definition 2 (D2), a ≥1-unit decrease in transition dyspnea index replaced FEV1.
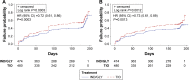
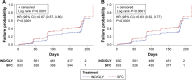
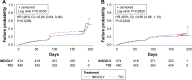
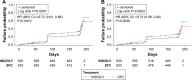
Results: Using D1, IND/GLY significantly reduced the risk of first or sustained CID versus either TIO (hazard ratio 0.72 [0.61, 0.86], P=0.0003 and 0.73 [0.61, 0.89], P=0.001) or SFC (0.67 [0.57, 0.80] and 0.63 [0.52, 0.77], both P<0.0001). With D2, IND/GLY significantly reduced the risk of first, but not sustained, CID versus TIO (0.80 [0.64 to 0.99], P=0.0359 and 0.85 [0.66, 1.10], P=0.2208) and both first and sustained CID versus SFC (0.73 [0.61, 0.88], P=0.001 and 0.72 [0.58, 0.90], P=0.0036).
Conclusion: These data confirm the utility of the CID endpoint as a means of monitoring COPD worsening in patients with moderate-to-severe COPD. Using the CID measure, we demonstrated that dual bronchodilation with IND/GLY significantly reduced the risk of CID versus either long-acting muscarinic antagonist or long-acting β2-agonist/inhaled corticosteroid treatment, providing further evidence for the benefit of dual bronchodilation in this patient population.
Keywords: COPD; IND/GLY; deterioration.
Conflict of interest statement
Disclosure The authors take full responsibility for the scope, direction, content, and editorial decisions relating to the manuscript, and were involved at all stages of development and approved the submitted manuscript. The authors received no compensation related to the development of the manuscript. ARA has acted as a consultant and served on advisory boards for Novartis Pharma AG, AstraZeneca, Boehringer Ingelheim, GSK, and Sunovion. CFV has acted as a consultant and served on advisory boards for Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi; received grants and personal fees from GlaxoSmithKline, grants and personal fees from Grifols, Mundipharma, Novartis, Takeda, Cipla, and Berlin Chemie/Menarini. KK is an employee and shareholder of Novartis Pharma AG. Previously he has received honoraria for educational activities and lectures from AstraZeneca, Boehringer Ingelheim, Chiesi, ELPEN, GSK, Novartis, and Takeda, and has participated on advisory boards arranged by AstraZeneca, Chiesi, ELPEN, Novartis, and Takeda. KM, SF, GB, SS, DB and RF are employees and shareholders of Novartis Pharma AG or Novartis Pharmaceuticals Corporation. The authors have no other conflicts of interest to report in this work.
Figures


References
-
- Vogelmeier CF, Bateman ED, Pallante J, et al. Efficacy and safety of once-daily QVA149 compared with twice-daily salmeterol-fluticasone in patients with chronic obstructive pulmonary disease (ILLUMI NATE): a randomised, double-blind, parallel group study. Lancet Respir Med. 2013;1(1):51–60. - PubMed
-
- goldcopd.org [homepage on the Internet] Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD); as current in 2017. [Accessed March 26, 2017]. Available from: http://goldcopd.org.
-
- Celli BR, Thomas NE, Anderson JA, et al. Effect of pharmacotherapy on rate of decline of lung function in chronic obstructive pulmonary disease: results from the TORCH study. Am J Respir Crit Care Med. 2008;178(4):332–338. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

