Is periodontitis a comorbidity of COPD or can associations be explained by shared risk factors/behaviors?
- PMID: 28496317
- PMCID: PMC5422335
- DOI: 10.2147/COPD.S127802
Is periodontitis a comorbidity of COPD or can associations be explained by shared risk factors/behaviors?
Abstract
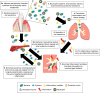
COPD is recognized as having a series of comorbidities potentially related to common inflammatory processes. Periodontitis is one of the most common human inflammatory diseases and has previously been associated with COPD in numerous observational studies. As periodontitis and COPD are both chronic, progressive conditions characterized by neutrophilic inflammation with subsequent proteolytic destruction of connective tissue, it has been proposed that they share common pathophysiological processes. The mechanisms proposed to link COPD and periodontitis include mechanical aspiration of oral contents into the respiratory tree, overspill of locally produced inflammatory mediators into the systemic circulation or oral or lung-derived bacteremia activating an acute-phase response and also reactive oxygen species (ROS) and cytokine release by systemic neutrophils at distant sites. Studies of systemic neutrophils in COPD and chronic periodontitis describe altered cellular functions that would predispose to inflammation and tissue destruction both in the lung and in the mouth, again potentially connecting these conditions. However, COPD and periodontitis also share risk factors such as age, chronic tobacco smoke exposure, and social deprivation that are not always considered in observational and interventional studies. Furthermore, studies reporting associations have often utilized differing definitions of both COPD and periodontitis. This article reviews the current available evidence supporting the hypothesis that COPD and inflammatory periodontal disease (periodontitis) could be pathologically associated, including a review of shared inflammatory mechanisms. It highlights the potential limitations of previous studies, in particular, the lack of uniformly applied case definitions for both COPD and periodontitis and poor recognition of shared risk factors. Understanding associations between these conditions may inform why patients with COPD suffer such a burden of comorbid illness and new therapeutic strategies for both the diseases. However, further research is needed to clarify factors that may be directly causal as opposed to confounding relationships.
Keywords: dental health; emphysema; inflammation; neutrophil; smoking.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures

Similar articles
-
Review of the association between periodontitis and chronic obstructive pulmonary disease in smokers.Monaldi Arch Chest Dis. 2019 Mar 20;89(1). doi: 10.4081/monaldi.2019.1018. Monaldi Arch Chest Dis. 2019. PMID: 30968666 Review.
-
The clinical and inflammatory relationships between periodontitis and chronic obstructive pulmonary disease.J Clin Periodontol. 2020 Sep;47(9):1040-1052. doi: 10.1111/jcpe.13334. Epub 2020 Jul 9. J Clin Periodontol. 2020. PMID: 32567697
-
Research on the Association Between Periodontitis and COPD.Int J Chron Obstruct Pulmon Dis. 2023 Sep 1;18:1937-1948. doi: 10.2147/COPD.S425172. eCollection 2023. Int J Chron Obstruct Pulmon Dis. 2023. PMID: 37675198 Free PMC article. Review.
-
Shared mechanisms of multimorbidity in COPD, atherosclerosis and type-2 diabetes: the neutrophil as a potential inflammatory target.Eur Respir Rev. 2020 Mar 20;29(155):190102. doi: 10.1183/16000617.0102-2019. Print 2020 Mar 31. Eur Respir Rev. 2020. PMID: 32198215 Free PMC article. Review.
-
Periodontal disease increases the severity of chronic obstructive pulmonary disease: a Mendelian randomization study.BMC Pulm Med. 2024 May 3;24(1):220. doi: 10.1186/s12890-024-03025-6. BMC Pulm Med. 2024. PMID: 38702679 Free PMC article.
Cited by
-
Association between periodontitis and peripheral artery disease: a systematic review and meta-analysis.BMC Cardiovasc Disord. 2018 Jul 6;18(1):141. doi: 10.1186/s12872-018-0879-0. BMC Cardiovasc Disord. 2018. PMID: 29980169 Free PMC article.
-
Porphyromonas gingivalis inducing autophagy-related biological dysfunction in alveolar epithelial cells: an in vitro study.BMC Oral Health. 2024 Dec 5;24(1):1478. doi: 10.1186/s12903-024-05253-y. BMC Oral Health. 2024. PMID: 39639253 Free PMC article.
-
Associations between Periodontitis and COPD: An Artificial Intelligence-Based Analysis of NHANES III.J Clin Med. 2022 Dec 4;11(23):7210. doi: 10.3390/jcm11237210. J Clin Med. 2022. PMID: 36498784 Free PMC article.
-
Periodontal Pathogens as Risk Factors of Cardiovascular Diseases, Diabetes, Rheumatoid Arthritis, Cancer, and Chronic Obstructive Pulmonary Disease-Is There Cause for Consideration?Microorganisms. 2019 Oct 9;7(10):424. doi: 10.3390/microorganisms7100424. Microorganisms. 2019. PMID: 31600905 Free PMC article. Review.
-
The Role of Interleukin 6 in Periodontitis and Its Complications.Int J Mol Sci. 2024 Feb 10;25(4):2146. doi: 10.3390/ijms25042146. Int J Mol Sci. 2024. PMID: 38396821 Free PMC article. Review.
References
-
- Sapey E, Bayley D, Ahmad A, Newbold P, Snell N, Stockley RA. Inter-relationships between inflammatory markers in stable COPD patients with bronchitis; the intra and inter patient variability. Thorax. 2008;63:493–499. - PubMed
-
- Stockley RA. Neutrophils and the pathogenesis of COPD. Chest. 2002;121:151S–155S. - PubMed
-
- Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. 2015. [Accessed January 14, 2017]. Available from: http://goldcopd.org/
-
- Vanfleteren LE, Spruit MA, Groenen M, et al. Clusters of comorbidities based on validated objective measurements and systemic inflammation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;187(7):728–735. - PubMed
-
- Mapel DW, Hurley JS, Frost FJ, Petersen HV, Picchi MA, Coultas DB. Health care utilization in chronic obstructive pulmonary disease: a case-control study in a health maintenance organization. Arch Intern Med. 2000;160(17):2653–2658. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

