A novel nanoemulsion-based method to produce ultrasmall, water-dispersible nanoparticles from chitosan, surface modified with cell-penetrating peptide for oral delivery of proteins and peptides
- PMID: 28496323
- PMCID: PMC5422456
- DOI: 10.2147/IJN.S116063
A novel nanoemulsion-based method to produce ultrasmall, water-dispersible nanoparticles from chitosan, surface modified with cell-penetrating peptide for oral delivery of proteins and peptides
Retraction in
-
A Novel Nanoemulsion-Based Method to Produce Ultrasmall, Water-Dispersible Nanoparticles from Chitosan, Surface Modified with Cell-Penetrating Peptide for Oral Delivery of Proteins and Peptides [Retraction].Int J Nanomedicine. 2022 Mar 29;17:1461-1462. doi: 10.2147/IJN.S367798. eCollection 2022. Int J Nanomedicine. 2022. PMID: 35378883 Free PMC article.
Abstract
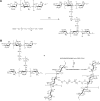
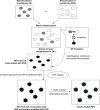
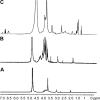
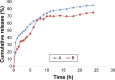
A simple and reproducible water-in-oil (W/O) nanoemulsion technique for making ultrasmall (<15 nm), monodispersed and water-dispersible nanoparticles (NPs) from chitosan (CS) is reported. The nano-sized (50 nm) water pools of the W/O nanoemulsion serve as "nano-containers and nano-reactors". The entrapped polymer chains of CS inside these "nano-reactors" are covalently cross-linked with the chains of polyethylene glycol (PEG), leading to rigidification and formation of NPs. These NPs possess excessive swelling properties in aqueous medium and preserve integrity in all pH ranges due to chemical cross-linking with PEG. A potent and newly developed cell-penetrating peptide (CPP) is further chemically conjugated to the surface of the NPs, leading to development of a novel peptide-conjugated derivative of CS with profound tight-junction opening properties. The CPP-conjugated NPs can easily be loaded with almost all kinds of proteins, peptides and nucleotides for oral delivery applications. Feasibility of this nanoparticulate system for oral delivery of a model peptide (insulin) is investigated in Caco-2 cell line. The cell culture results for translocation of insulin across the cell monolayer are very promising (15%-19% increase), and animal studies are actively under progress and will be published separately.
Keywords: Caco-2 cell; cell-penetrating peptide; chitosan; nanoemulsion; oral insulin; ultrasmall.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures






Similar articles
-
Modified nanoparticles with cell-penetrating peptide and amphipathic chitosan derivative for enhanced oral colon absorption of insulin: preparation and evaluation.Drug Deliv. 2016 Jul;23(6):2003-14. doi: 10.3109/10717544.2015.1048489. Epub 2015 Jul 16. Drug Deliv. 2016. PMID: 26181840
-
Synthesis and characterization of a novel peptide-grafted Cs and evaluation of its nanoparticles for the oral delivery of insulin, in vitro, and in vivo study.Int J Nanomedicine. 2018 Sep 6;13:5127-5138. doi: 10.2147/IJN.S161240. eCollection 2018. Int J Nanomedicine. 2018. Retraction in: Int J Nanomedicine. 2020 Mar 09;15:1623. doi: 10.2147/IJN.S252407. PMID: 30233176 Free PMC article. Retracted.
-
Development and characterisation of chitosan films impregnated with insulin loaded PEG-b-PLA nanoparticles (NPs): a potential approach for buccal delivery of macromolecules.Int J Pharm. 2012 May 30;428(1-2):143-51. doi: 10.1016/j.ijpharm.2012.02.035. Epub 2012 Mar 1. Int J Pharm. 2012. PMID: 22405987
-
Chitosan Nanoparticles as a Novel Drug Delivery System: A Review Article.Curr Drug Targets. 2020;21(15):1613-1624. doi: 10.2174/1389450121666200711172536. Curr Drug Targets. 2020. PMID: 32651965 Review.
-
Interaction of nanoparticles and cell-penetrating peptides with skin for transdermal drug delivery.Mol Membr Biol. 2010 Oct;27(7):247-59. doi: 10.3109/09687688.2010.522203. Mol Membr Biol. 2010. PMID: 21028936 Free PMC article. Review.
Cited by
-
Efficiency of GnRH-Loaded Chitosan Nanoparticles for Inducing LH Secretion and Fertile Ovulations in Protocols for Artificial Insemination in Rabbit Does.Animals (Basel). 2021 Feb 8;11(2):440. doi: 10.3390/ani11020440. Animals (Basel). 2021. PMID: 33567711 Free PMC article.
-
Impact Of Penetratin Stereochemistry On The Oral Bioavailability Of Insulin-Loaded Solid Lipid Nanoparticles.Int J Nanomedicine. 2019 Nov 25;14:9127-9138. doi: 10.2147/IJN.S225086. eCollection 2019. Int J Nanomedicine. 2019. PMID: 31819423 Free PMC article.
-
Overcoming Multiple Absorption Barrier for Insulin Oral Delivery Using Multifunctional Nanoparticles Based on Chitosan Derivatives and Hyaluronic Acid.Int J Nanomedicine. 2020 Jul 9;15:4877-4898. doi: 10.2147/IJN.S251627. eCollection 2020. Int J Nanomedicine. 2020. PMID: 32753869 Free PMC article.
-
Advances in Oral Drug Delivery Systems: Challenges and Opportunities.Pharmaceutics. 2023 Feb 1;15(2):484. doi: 10.3390/pharmaceutics15020484. Pharmaceutics. 2023. PMID: 36839807 Free PMC article. Review.
-
Multifunctional oral delivery systems for enhanced bioavailability of therapeutic peptides/proteins.Acta Pharm Sin B. 2019 Sep;9(5):902-922. doi: 10.1016/j.apsb.2019.01.004. Epub 2019 Jan 10. Acta Pharm Sin B. 2019. PMID: 31649842 Free PMC article. Review.
References
-
- Moroz E, Matoori S, Leroux J-C. Oral delivery of macromolecular drugs: where we are after almost 100 years of attempts. Adv Drug Deliv Rev. 2016;101:108–121. - PubMed
-
- Khafagy E-S, Morishita M, Onuki Y, Takayama K. Current challenges in non-invasive insulin delivery systems: a comparative review. Adv Drug Deliv Rev. 2007;59(15):1521–1546. - PubMed
-
- Sousa F, Castro P, Fonte P, Sarmento B. How to overcome the limitations of current insulin administration with new non-invasive delivery systems. Ther Deliv. 2015;6(1):83–94. - PubMed
-
- He H, Ye J, Sheng J, et al. Overcoming oral insulin delivery barriers: application of cell penetrating peptide and silica-based nanoporous composites. Front Chem Sci Eng. 2013;7(1):9–19.
-
- Ross P, Milburn J, Reith D, Wiltshire E, Wheeler B. Clinical review: insulin pump-associated adverse events in adults and children. Acta Diabetol. 2015;52(6):1017–1024. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

