Efficacy and safety of a flexible extended regimen of ethinylestradiol/drospirenone for the treatment of dysmenorrhea: a multicenter, randomized, open-label, active-controlled study
- PMID: 28496369
- PMCID: PMC5422539
- DOI: 10.2147/IJWH.S134576
Efficacy and safety of a flexible extended regimen of ethinylestradiol/drospirenone for the treatment of dysmenorrhea: a multicenter, randomized, open-label, active-controlled study
Abstract
Background: Dysmenorrhea is a common condition in women, which is characterized by menstrual pain. Low-dose estrogen/progestin combined oral contraceptives have been shown to reduce the severity of dysmenorrhea symptoms, and a 28-day cyclic regimen of ethinylestradiol/drospirenone (28d regimen) is approved for this indication in Japan.
Aim: The aim of this study was to assess the safety and efficacy of a flexible extended regimen of ethinylestradiol/drospirenone (flexible regimen) in Japanese women with dysmenorrhea.
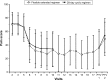
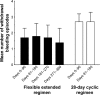
Methods: This multicenter, open-label study was performed in Japanese women with dysmenorrhea who, after a baseline observational phase, were randomized to receive ethinylestradiol 20 μg/drospirenone 3 mg in a flexible regimen (one tablet each day for 24-120 days followed by a 4-day tablet-free interval) or in the standard 28d regimen (one tablet each day for 24 days, followed by 4 days of placebo tablets for six cycles). The primary endpoint was the number of days with dysmenorrhea of at least mild intensity over a 140-day evaluation period. Dysmenorrhea scores, bleeding patterns, and other pain-related parameters were also assessed.
Results: A total of 216 women (mean age 29.7 years) were randomized to the flexible regimen (n=108) or 28d regimen (n=108) and 212 were included in the full analysis sets (flexible regimen, n=105; 28d regimen, n=107). Women in the flexible-regimen group reported a mean of 3.4 fewer days with dysmenorrheic pain than women in the 28d-regimen group, with similar decreases in disease severity reported in both treatment groups. According to the investigators, 64.8% and 59.4% of women in the flexible-regimen and 28d-regimen treatment groups had "very much improved" or "much improved" disease, while 54.3% and 50.9% of patients reported being "very much satisfied" or "much satisfied" with their treatment, respectively.
Conclusion: In Japanese women with dysmenorrhea, a flexible extended regimen of ethinylestradiol/drospirenone decreased the number of days with dysmenorrheic pain versus the traditional 28d regimen.
Keywords: dysmenorrhea; ethinylestradiol/drospirenone; flexible extended regimen; oral contraceptive; pain relief.
Conflict of interest statement
Disclosure The sponsor, Bayer Yakuhin Ltd, provided financial support for the conduct of the research and preparation of the article. The sponsor designed and conducted the study including collection, management, and analysis of data. Mikio Momoeda and Tasuku Harada received a medical advisory fee from Bayer Yakuhin Ltd when the study was planned and conducted. Masami Kondo, Masanobu Yasuda, and Shigetomo Yamamoto are employees of Bayer Yakuhin Ltd and Joerg Elliesen is an employee of Bayer Pharma AG. The authors report no other conflicts of interest in this work.
Figures


References
-
- Deligeoroglou E. Dysmenorrhea. Ann N Y Acad Sci. 2000;900:237–244. - PubMed
-
- Ju H, Jones M, Mishra G. The prevalence and risk factors of dysmenorrhea. Epidemiol Rev. 2014;36:104–113. - PubMed
-
- Teperi J, Rimpela M. Menstrual pain, health and behaviour in girls. Soc Sci Med. 1989;29(2):163–169. - PubMed
-
- Tanaka E, Momoeda M, Osuga Y, et al. Burden of menstrual symptoms in Japanese women: results from a survey-based study. J Med Econ. 2013;16(11):1255–1266. - PubMed
-
- Weissman AM, Hartz AJ, Hansen MD, Johnson SR. The natural history of primary dysmenorrhoea: a longitudinal study. BJOG. 2004;111(4):345–352. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

