The Impact of Microbiota-Gut-Brain Axis on Diabetic Cognition Impairment
- PMID: 28496408
- PMCID: PMC5406474
- DOI: 10.3389/fnagi.2017.00106
The Impact of Microbiota-Gut-Brain Axis on Diabetic Cognition Impairment
Abstract
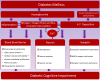
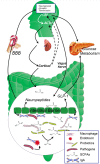
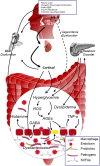
Progressive cognitive dysfunction is a central characteristic of diabetic encephalopathy (DE). With an aging population, the incidence of DE is rising and it has become a major threat that seriously affects public health. Studies within this decade have indicated the important role of risk factors such as oxidative stress and inflammation on the development of cognitive impairment. With the recognition of the two-way communication between gut and brain, recent investigation suggests that "microbiota-gut-brain axis" also plays a pivotal role in modulating both cognition function and endocrine stability. This review aims to systemically elucidate the underlying impact of diabetes on cognitive impairment.
Keywords: advanced glycation end products; diabetic encephalopathy; gut; hypothalamic-pituitary-adrenal axis; inflammation; microbiota.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

