Lysophosphatidic Acid Is Associated with Atherosclerotic Plaque Instability by Regulating NF-κB Dependent Matrix Metalloproteinase-9 Expression via LPA2 in Macrophages
- PMID: 28496416
- PMCID: PMC5406459
- DOI: 10.3389/fphys.2017.00266
Lysophosphatidic Acid Is Associated with Atherosclerotic Plaque Instability by Regulating NF-κB Dependent Matrix Metalloproteinase-9 Expression via LPA2 in Macrophages
Abstract
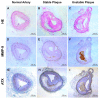
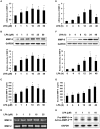
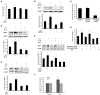
Lysophosphatidic acid (LPA), one of the simplest phospholipid signaling molecules, participates in formation and disruption of atherosclerotic plaque. Matrix metalloproteinases (MMPs) contribute to atherosclerotic plaque rupture by involving in extracellular matrix (ECM) degradation and then thinning fibrous cap. Our previous study demonstrated that macrophage-derived MMP-9 was associated with coronary plaque instability, but the relationship between LPA and MMP-9 remains unclear. The present work therefore aimed at elucidating association between LPA and MMP-9 and the regulation mechanism of LPA on MMP-9 in macrophages. We found that plasma LPA and MMP-9 levels were correlated positively (r = 0.31, P < 0.05) and both elevated significantly in patients with acute myocardial infarct (AMI). Consistent with peripheral blood levels, histochemical staining indicated that autotaxin (ATX), LPA-producing ectoenzyme, and MMP-9 were expressed frequently in the necrotic core and fibrous cap of human unstable plaques, which might increase the instability of plaque. Experiments in vitro were done with THP-1-derived macrophages and showed that LPA enhanced the expression, secretion and activity of MMP-9 in a time- and dose-dependent manner. Induction of LPA on pro-MMP-9 and active-MMP-9 was confirmed in human peripheral blood monocyte-derived macrophages. PDTC, NF-κB inhibitor, but not inhibitor of AP-1 and PPARγ, effectively prevented LPA-induced MMP-9 expression and NF-κB p65 siRNA decreased MMP-9 transcription, confirming that LPA might induce MMP-9 elevation by activating NF-κB pathway. In addition, knockdown of LPA2 attenuated LPA-induced MMP-9 expression and nucleus p65 levels. These findings revealed that LPA upregulated the expression of MMP-9 through activating NF-κB pathway in the LPA2 dependent manner, hence blocking LPA receptors signaling may provide therapeutic strategy to target plaque destabilization.
Keywords: LPA2; coronary atherosclerotic plaques; lysophosphatidic acid; macrophages; matrix metalloproteinase-9.
Figures


References
-
- Blankenberg S., Rupprecht H. J., Poirier O., Bickel C., Smieja M., Hafner G., et al. . (2003). Plasma concentrations and genetic variation of matrix metalloproteinase 9 and prognosis of patients with cardiovascular disease. Circulation 107, 1579–1585. 10.1161/01.CIR.0000058700.41738.12 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

