Cholinergic Nociceptive Mechanisms in Rat Meninges and Trigeminal Ganglia: Potential Implications for Migraine Pain
- PMID: 28496430
- PMCID: PMC5406407
- DOI: 10.3389/fneur.2017.00163
Cholinergic Nociceptive Mechanisms in Rat Meninges and Trigeminal Ganglia: Potential Implications for Migraine Pain
Abstract
Background: Parasympathetic innervation of meninges and ability of carbachol, acetylcholine (ACh) receptor (AChR) agonist, to induce headaches suggests contribution of cholinergic mechanisms to primary headaches. However, neurochemical mechanisms of cholinergic regulation of peripheral nociception in meninges, origin place for headache, are almost unknown.
Methods: Using electrophysiology, calcium imaging, immunohistochemistry, and staining of meningeal mast cells, we studied effects of cholinergic agents on peripheral nociception in rat hemiskulls and isolated trigeminal neurons.
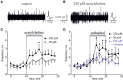
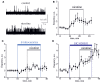
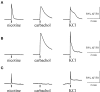
Results: Both ACh and carbachol significantly increased nociceptive firing in peripheral terminals of meningeal trigeminal nerves recorded by local suction electrode. Strong nociceptive firing was also induced by nicotine, implying essential role of nicotinic AChRs in control of excitability of trigeminal nerve endings. Nociceptive firing induced by carbachol was reduced by muscarinic antagonist atropine, whereas the action of nicotine was prevented by the nicotinic blocker d-tubocurarine but was insensitive to the TRPA1 antagonist HC-300033. Carbachol but not nicotine induced massive degranulation of meningeal mast cells known to release multiple pro-nociceptive mediators. Enzymes terminating ACh action, acetylcholinesterase (AChE) and butyrylcholinesterase, were revealed in perivascular meningeal nerves. The inhibitor of AChE neostigmine did not change the firing per se but induced nociceptive activity, sensitive to d-tubocurarine, after pretreatment of meninges with the migraine mediator CGRP. This observation suggested the pro-nociceptive action of endogenous ACh in meninges. Both nicotine and carbachol induced intracellular Ca2+ transients in trigeminal neurons partially overlapping with expression of capsaicin-sensitive TRPV1 receptors.
Conclusion: Trigeminal nerve terminals in meninges, as well as dural mast cells and trigeminal ganglion neurons express a repertoire of pro-nociceptive nicotinic and muscarinic AChRs, which could be activated by the ACh released from parasympathetic nerves. These receptors represent a potential target for novel therapeutic interventions in trigeminal pain and probably in migraine.
Keywords: acetylcholine; acetylcholine receptor; mast cells; meninges; migraine; nicotine; sensory neurons.
Figures





Comment in
-
Commentary: Cholinergic Nociceptive Mechanisms in Rat Meninges and Trigeminal Ganglia: Potential Implications for Migraine Pain.Front Neurol. 2017 Nov 21;8:623. doi: 10.3389/fneur.2017.00623. eCollection 2017. Front Neurol. 2017. PMID: 29276497 Free PMC article. No abstract available.
Similar articles
-
Serotonergic mechanisms of trigeminal meningeal nociception: Implications for migraine pain.Neuropharmacology. 2017 Apr;116:160-173. doi: 10.1016/j.neuropharm.2016.12.024. Epub 2016 Dec 23. Neuropharmacology. 2017. PMID: 28025094
-
Receptor Mechanisms Mediating the Pro-Nociceptive Action of Hydrogen Sulfide in Rat Trigeminal Neurons and Meningeal Afferents.Front Cell Neurosci. 2017 Jul 27;11:226. doi: 10.3389/fncel.2017.00226. eCollection 2017. Front Cell Neurosci. 2017. PMID: 28798669 Free PMC article.
-
Mechanosensitive meningeal nociception via Piezo channels: Implications for pulsatile pain in migraine?Neuropharmacology. 2019 May 1;149:113-123. doi: 10.1016/j.neuropharm.2019.02.015. Epub 2019 Feb 13. Neuropharmacology. 2019. PMID: 30768945
-
Parasympathetic Cholinergic and Neuropeptide Mechanisms of Migraine.Anesth Pain Med. 2016 Dec 18;7(1):e42210. doi: 10.5812/aapm.42210. eCollection 2017 Feb. Anesth Pain Med. 2016. PMID: 28920040 Free PMC article. Review.
-
Sex difference in TRPM3 channel functioning in nociceptive and vascular systems: an emerging target for migraine therapy in females?J Headache Pain. 2025 Feb 24;26(1):40. doi: 10.1186/s10194-025-01966-9. J Headache Pain. 2025. PMID: 39994546 Free PMC article. Review.
Cited by
-
Should "Retro-ocular Pain, Photophobia and Visual Acuity Loss" Be Recognised as a Distinct Entity? The ROPPVAL Syndrome.Neuroophthalmology. 2021 May 3;45(4):253-260. doi: 10.1080/01658107.2021.1887289. eCollection 2021. Neuroophthalmology. 2021. PMID: 34366513 Free PMC article.
-
Investigating risk factors for migraine in Syrian women: a cross-sectional case-control study.Sci Rep. 2025 Feb 3;15(1):4148. doi: 10.1038/s41598-025-87487-6. Sci Rep. 2025. PMID: 39900976 Free PMC article.
-
Effect of Smoking on the Development of Migraine in Women: Nationwide Cohort Study in South Korea.JMIR Public Health Surveill. 2024 Aug 23;10:e58105. doi: 10.2196/58105. JMIR Public Health Surveill. 2024. PMID: 39177651 Free PMC article.
-
Inhibiting Endocannabinoid Hydrolysis as Emerging Analgesic Strategy Targeting a Spectrum of Ion Channels Implicated in Migraine Pain.Int J Mol Sci. 2022 Apr 15;23(8):4407. doi: 10.3390/ijms23084407. Int J Mol Sci. 2022. PMID: 35457225 Free PMC article. Review.
-
Protective Effects of Hydrogen Sulfide Against the ATP-Induced Meningeal Nociception.Front Cell Neurosci. 2020 Sep 2;14:266. doi: 10.3389/fncel.2020.00266. eCollection 2020. Front Cell Neurosci. 2020. PMID: 32982692 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

