Convergent synthesis and optical properties of near-infrared emitting bioluminescent infra-luciferins
- PMID: 28496975
- PMCID: PMC5361108
- DOI: 10.1039/c6ra19541e
Convergent synthesis and optical properties of near-infrared emitting bioluminescent infra-luciferins
Abstract
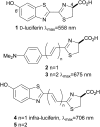
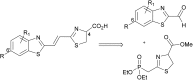
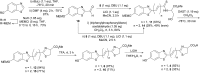
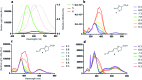
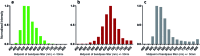
Infra-luciferin, an alkene linked analogue of luciferin, gives bioluminescence emission >700 nm and has the potential to be used for multiparametric in vivo imaging. We report here a high yielding, scalable and convergent synthesis of infra-luciferin which will allow the synthesis of other conjugated luciferins for investigation in near-infrared bioluminescence imaging. We demonstrated this potential by using the new route to synthesise a diene linked analogue of luciferin, the fluorescent and bioluminescent properties of which were compared to those of d-luciferin and infra-luciferin. We found that extension of conjugation to a diene linker resulted in the specific bioluminescence activity being reduced by 3-4 orders of magnitude compared to d-luciferin. Analogous to its fluorescence emission spectrum, the diene linked analogue exhibited two peaks in its bioluminescence spectrum, the major one being slightly blue-shifted compared to natural d-luciferin, and a minor peak at ca. 800 nm. The fluorescence quantum yield and pH dependence of fluorescence were also determined.
Figures

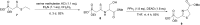

References
-
- Fan F., Wood K. V. Assay Drug Dev. Technol. 2007;5:127. - PubMed
-
- Prescher J. A., Contag C. H. Curr. Opin. Chem. Biol. 2010;14:80. - PubMed
-
- Ando Y., Niwa K., Yamada N., Enomoto T., Irie T., Kubota H., Ohmiya Y., Akiyama H. Nat. Photonics. 2008;2:44.
-
- De Almeida P. E., Van Rappard J. R. M., Wu J. C. Am. J. Physiol.: Heart Circ. Physiol. 2011;301:H663. - PMC - PubMed
- Sadikot R. T., Blackwell T. S. Methods Mol. Biol. 2008;477:383. - PubMed
- Duda J., Karimi M., Negrin R. S., Contag G. H. Methods Mol. Med. 2007;134:17. - PubMed
- Badr C. E., Tannous B. A. Trends Biotechnol. 2011;29:624. - PMC - PubMed
-
- Rice B. W., Cable M. D., Nelson M. B. J. Biomed. Opt. 2001;6:432. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
