A generalized quantitative antibody homeostasis model: antigen saturation, natural antibodies and a quantitative antibody network
- PMID: 28496977
- PMCID: PMC5333986
- DOI: 10.1038/cti.2016.90
A generalized quantitative antibody homeostasis model: antigen saturation, natural antibodies and a quantitative antibody network
Erratum in
-
Corrigendum: A generalized quantitative antibody homeostasis model: antigen saturation, natural antibodies and a quantitative antibody network.Clin Transl Immunology. 2017 May 19;6(5):e142. doi: 10.1038/cti.2017.19. eCollection 2017 May. Clin Transl Immunology. 2017. PMID: 28690848 Free PMC article.
Abstract
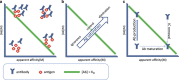
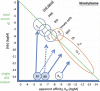
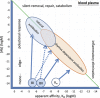
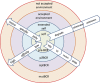
In a pair of articles, we present a generalized quantitative model for the homeostatic function of clonal humoral immune system. In this second paper, we describe how antibody production controls the saturation of antigens and the network of antibody interactions that emerges in the epitome space with the establishment of the immune system. Efficient control of antigens, be it self or foreign, requires the maintenance of antibody concentrations that saturate antigen to relevant levels. Simple calculations suggest that the observed diverse recognition of antigens by natural antibodies is only possible by cross-reactivity whereby particular clones of antibodies bind to diverse targets and shared recognition of particular antigens by multiple antibody clones contribute to the maintenance of antigen control. We also argue that natural antibodies are none else than the result of thymus-independent responses against immunological self. We interpret and explain antibody production and function in a virtual molecular interaction space and as a network of interactions. Indeed, the general quantitative (GQM) model we propose is in agreement with earlier models, confirms some assumptions and presumably provides the theoretical basis for the construction of a real antibody network using the sequence and interaction database data.
Conflict of interest statement
The author declares no conflict of interest.
Figures

References
-
- Seigneurin JM, Guilbert B, Bourgeat MJ, Avrameas S. Polyspecific natural antibodies and autoantibodies secreted by human lymphocytes immortalized with Epstein-Barr virus. Blood 1988; 71: 581–585. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
