Reciprocal Interactions between Nematodes and Their Microbial Environments
- PMID: 28497029
- PMCID: PMC5406411
- DOI: 10.3389/fcimb.2017.00144
Reciprocal Interactions between Nematodes and Their Microbial Environments
Abstract
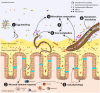
Parasitic nematode infections are widespread in nature, affecting humans as well as wild, companion, and livestock animals. Most parasitic nematodes inhabit the intestines of their hosts living in close contact with the intestinal microbiota. Many species also have tissue migratory life stages in the absence of severe systemic inflammation of the host. Despite the close coexistence of helminths with numerous microbes, little is known concerning these interactions. While the environmental niche is considerably different, the free-living nematode Caenorhabditis elegans (C. elegans) is also found amongst a diverse microbiota, albeit on decaying organic matter. As a very well characterized model organism that has been intensively studied for several decades, C. elegans interactions with bacteria are much more deeply understood than those of their parasitic counterparts. The enormous breadth of understanding achieved by the C. elegans research community continues to inform many aspects of nematode parasitology. Here, we summarize what is known regarding parasitic nematode-bacterial interactions while comparing and contrasting this with information from work in C. elegans. This review highlights findings concerning responses to bacterial stimuli, antimicrobial peptides, and the reciprocal influences between nematodes and their environmental bacteria. Furthermore, the microbiota of nematodes as well as alterations in the intestinal microbiota of mammalian hosts by helminth infections are discussed.
Keywords: antibiotic resistance; antimicrobial peptides; helminth; microbiota; nematode.
Figures

Similar articles
-
Interactions between the intestinal microbiome and helminth parasites.Parasite Immunol. 2016 Jan;38(1):5-11. doi: 10.1111/pim.12274. Parasite Immunol. 2016. PMID: 26345715 Free PMC article. Review.
-
Sequential Changes in the Host Gut Microbiota During Infection With the Intestinal Parasitic Nematode Strongyloides venezuelensis.Front Cell Infect Microbiol. 2019 Jun 25;9:217. doi: 10.3389/fcimb.2019.00217. eCollection 2019. Front Cell Infect Microbiol. 2019. PMID: 31293983 Free PMC article.
-
Helminth-Bacterial Interactions: Cause and Consequence.Trends Immunol. 2018 Sep;39(9):724-733. doi: 10.1016/j.it.2018.06.002. Epub 2018 Jun 22. Trends Immunol. 2018. PMID: 29941203 Review.
-
Helminth Microbiomes - A Hidden Treasure Trove?Trends Parasitol. 2019 Jan;35(1):13-22. doi: 10.1016/j.pt.2018.10.007. Epub 2018 Nov 29. Trends Parasitol. 2019. PMID: 30503365 Review.
-
Cuticle surface coat of plant-parasitic nematodes.Annu Rev Phytopathol. 2011;49:135-56. doi: 10.1146/annurev-phyto-121310-111406. Annu Rev Phytopathol. 2011. PMID: 21568702 Review.
Cited by
-
Infection with soil-transmitted helminths and their impact on coinfections.Front Parasitol. 2023 May 24;2:1197956. doi: 10.3389/fpara.2023.1197956. eCollection 2023. Front Parasitol. 2023. PMID: 39816832 Free PMC article. Review.
-
Behavioral Cooperation or Conflict of Human Intestinal Roundworms and Microbiomes: A Bio-Activity Perspective.Cells. 2025 Apr 7;14(7):556. doi: 10.3390/cells14070556. Cells. 2025. PMID: 40214509 Free PMC article. Review.
-
Effect of Management System on Fecal Microbiota in Arabian Horses: Preliminary Results.Vet Sci. 2025 Mar 28;12(4):309. doi: 10.3390/vetsci12040309. Vet Sci. 2025. PMID: 40284811 Free PMC article.
-
Microbiota and gut ultrastructure of Anisakis pegreffii isolated from stranded cetaceans in the Adriatic Sea.Parasit Vectors. 2019 Jul 30;12(1):381. doi: 10.1186/s13071-019-3636-z. Parasit Vectors. 2019. PMID: 31362767 Free PMC article.
-
A Novel Non-invasive Method to Detect RELM Beta Transcript in Gut Barrier Related Changes During a Gastrointestinal Nematode Infection.Front Immunol. 2019 Mar 12;10:445. doi: 10.3389/fimmu.2019.00445. eCollection 2019. Front Immunol. 2019. PMID: 30915083 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

